Calculate the refractive index using the GladstoneDale approach for orthoclase of Problem 15.9 using the chemical refractivities
Question:
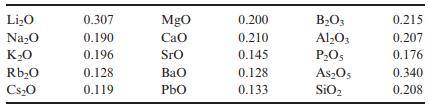
Calculate the refractive index using the Gladstone–Dale approach for orthoclase of Problem 15.9 using the chemical refractivities from Table 15.1. Compare the result with that of Problem 15.9.
Problem 15.9
Use the Anderson–Eggleton relationship to calculate the refractive index of the mineral orthoclase (KAlSi3O8) with a unit-cell volume 720.4 Å3 containing four formula units. The cation polarizabilities are 1.35 Å3, 0.533 Å3, and 0.284 Å3, for K+, Al3+, and Si4+, respectively. The free-ion polarizability α0 for oxygen is 1.79 Å3 and N0 is 1.776 Å3.
Table 15.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





