Question: A 0.400 kg sample is placed in a cooling apparatus that removes energy as heat at a constant rate. Figure gives the temperature T of
A 0.400 kg sample is placed in a cooling apparatus that removes energy as heat at a constant rate. Figure gives the temperature T of the sample versus time t; the horizontal scale is set by ts = 80.0 min. The sample freezes during the energy removal. The specific heat of the sample in its initial liquid phase is 3000 J/kg ? K. What are? br>
(a) The sample's heat of fusion and br>
(b) Its specific heat in the frozen phase?br>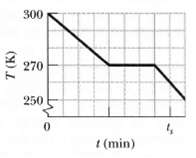
300 270 250 I (min) (N)
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
While the sample is in its liquid phase its temperature change in ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-T (339).docx
120 KBs Word File


