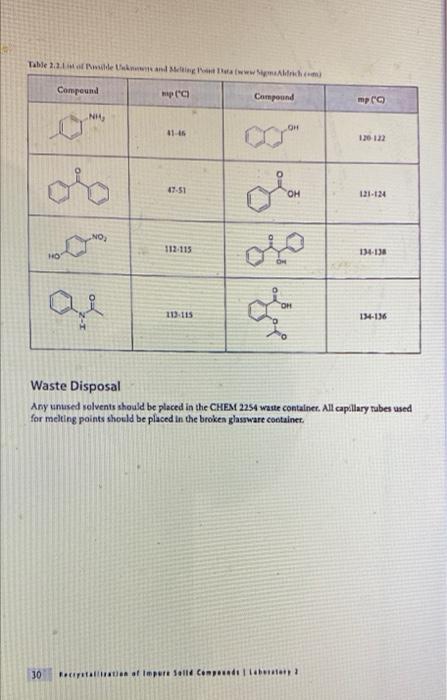
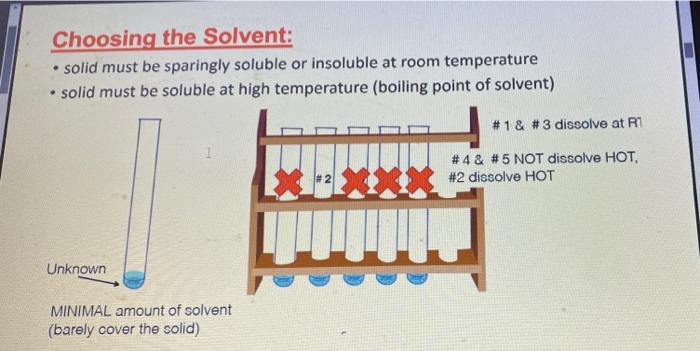
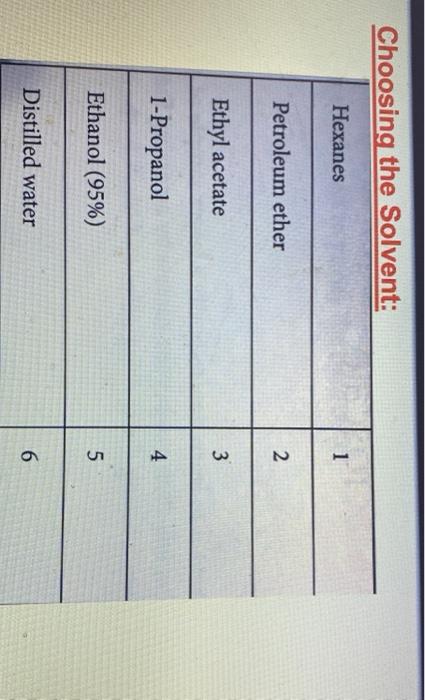
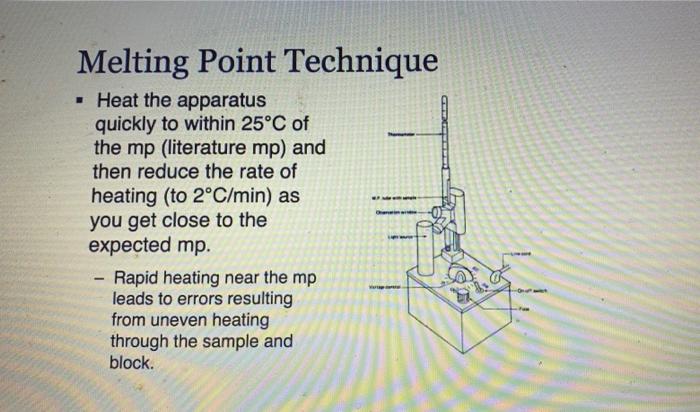
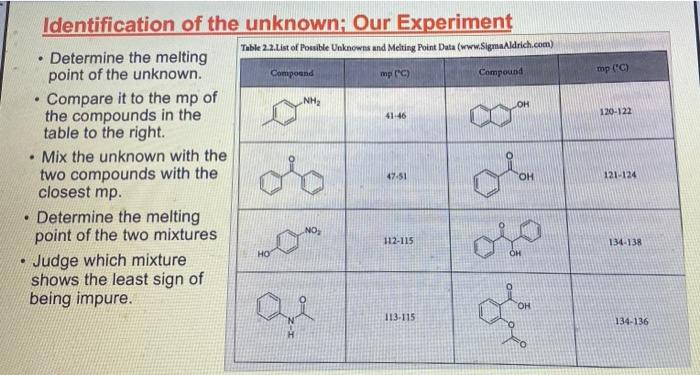
Experiment 2: Recrystallization of an Unknown-Macroscale Purpose The purpose of this experiment is to learn how to purify a larger sample of a crystalline compound through recrystallization. You will then gange your success by using the purity of your tecrystallized compound by measuring the melting point and identify the compound by and melting point . . Techniques Keeping a Lab Notebook Safety Pipets Cleaning and Drying Glassware Melting Points Recrystallization - . Assignments 1. Notebook preparation is required before lah. 2. Complete and submit before the assigned due date .. Online Pre-Lab Exercise: Recrystallization of an Unknown - Macroscale 3. Complete and submit by the start of the next lab period 2. Report Sheet: Recrystallization of an Unknown - Macroscale b. Online Post-Lab Questions Recrystallization of an Unknown - Macroscale Procedure Choosing the Solvent 1. Heat a 250-ml. beaker (or larger) of water on your hot plate. Add a couple of boiling stones. Check the temperature using a thermometer. You will want to initially heat the water to a temperature that is just above your LOWEST hosting solvent. 2. Obtain 1.3 g of an unknown sample. Record the code in your notebook Laban Recipale alipene Sol Cepat 22 3. Obtain 6 test tubes and label each with numbers 1-6. These number crepond to the went listed below Ines leche 2 lette 1-Prepanel thanol 5 Distilled water 4. Add approximately 0.10 g of your unknows to cach test tube and add 2 mL of the appropriate solvent. Shake the test tube to mix. If the solid dissolves at room temperature, put that sample aside, this solvent is NOT a good choice. If the solid does not dissolve at room temperature move on to step 5. Record this observation in your notebook 5. Take the samples still containing solid and suspend them in the hot water bath. Once the solvent inside the test tube is gently boiling, determine if the sold sample has disolved partially, completely, or not at all. If the solid has not dissolved at all remove this sample from the heat and set aside this solvent is NOT a good choice. Record this observation in your notebook 6. For the samples that are partially disolved, add additional solvent (you may wish to agitate the sample at this time) until the solid dissolves completely 7. Once your solid has completely dissolved with heating, remove the test tubes from the hot water bath and allow the lest tubes and contents to slowly cool to room temperature undisturbed. Do you see any crystal formation Record your observations 8. Once the test tube and contents have reached room temperature, carefully place the test tube in an ice bath for 10 min. Do you see any crystal formation Record your observatione 9. After the 10 min of cooling, examine the contents of the test tubes. Choose the solvent that resulted in the formation of the best crystals in the largest quantity. Record your solvent choice and reasoning in your notebook Recrystallization 10. Weigh the remaining solid unknown, record the weight in your notebook, and place all of the solid in a 50 mL Erlenmeyer flask. Add 1-2 bolling stones. Be sure to remove these latert 11. Place approximately 50 ml. of your chosen solvent in a 125-ml. Edenmeyer flask, wdd 1-2 boiling stones, and heat the solvent on your hot plate to a gentle boll Be careful to not heat the solvent foo hight You do not want the solvent to evaporale or ignite! 28 Recepten af impure Salle Constablety 12. Once the show is ingamell putin of the hot went to them. Firmy Hack with you holunni Parthen the plate small portion of bra, ing are the tank to the winntally down in the I went. It ha praticato in the latents of we do drugodt at the The idea is to relve your lil in a lot when the maintaining cometant volume. If your solent bookstovigortaly, the sevent will event and your lid will drive Remember. DISSOLVING is not the same 2 MELTING Y Joust want your sedal to melt or "oilout", leading to an impure sample. 13. Once the solid is completely divolved, remove the Erlenmeyer flask from the hot plate and allow it to cool SLOWLY to room temperature, undirbel 14. After you observe the formation of crystals, and the task has cooled all the way to room temperature cool the Erlenmeyer flank in an ice bath in order to maximize the amount of product that crystallines from the solution 15. Once you have cooled the Erlenmeyer flask in an ice bath for approximately 10 minutes isolate your crystals from the mother liquor by vacuum filtration. You should use a MINIMAL amount of ICE COLD recrystallizing solvent to transfer the remaining crystals . The crystals can then be washed with a MINIMAL amount of ICE COLD recrystallizing solvent. Room lemperature solvent will dissolve your crystals. 16. After allowing the crystals to dry, remove the boiling stones with forceps, measure the weight and record it in your notebook. Calculate the percent recovery and record it in your notebook 17. Obtain the melting point of your recrystallized and hopefully pure, unknown solid. Record the melting point range in your notebook 18. Compare your melting point to those listed in Table 32 of possible unknowns. Choose one compound from Table 3.2 and obtain a sample of the known compound from the reagents cart 19. You should now obtain a mixed melting point by grinding equal parts of your purified unknown and the known compound. A mortar and pestle is the best way to prepare the sample. Be sure to mix these compounds well! 20. Obtain the mixed melting point of the sample prepared in step 19. Record this value in your notebook. If the mixed melting point range is lower and broader than the melting point range for your pure compound, then the two compounds are not the same. If the mixed melting point range is identical (or nearly so) to that of your pure compound, then the two compounds are the same. 21. Using the mixed melting point data, correctly identify your unknown. Record this information 22. Place your purified sample in a glass vial, label it with your name, date, name of compound. and the measured melting point, and give it to your instructor In your notebook The Recitallaties ar lapure Salud Comprends Talle 2.2. Unawww Compound mpio Compound mp" NH OH 41-46 120 122 47.51 Ceo 121.124 NO 112-115 HO 114-130 113-115 134-136 Waste Disposal Any unused velvents should be placed in the CHEM 2254 waste contalter. All capillary tubes used for melting points should be placed in the broken glassware container. 30 Recitallisten af lepuresell Compensator Name Instructor Date Section Group Report Sheet: Laboratory 2b Recrystallization of Impure Solid Compounds: Experiment 2 PRE-LAB: The notebook pre-lah that you terned in prior to conducting the experiment DATA AND OBSERVATIONS Attach the carbon copy of your data and observations to the reportshert Part 1: Results 1. Unknown Code 2. Solvent choice for recrystallation z Fill in the following table and provide a brief statement for your choice of solvent in the space following the table See Sorrent tamilater (stroom temperature in terrains of pure Seile CINE 31 Percent recovery of un 4. Melting point of your pare unknown 3. Mixed melding point (mp) of your mixture Compound mixed with unknown Observed 6. Identity of your unknown (name and structure 32 Kristallation of impuresell Compounds Lantur Choosing the Solvent: solid must be sparingly soluble or insoluble at room temperature solid must be soluble at high temperature (boiling point of solvent) #1 & #3 dissolve at R7 1 #4& #5 NOT dissolve HOT. #2 dissolve HOT Unknown MINIMAL amount of solvent (barely cover the solid) . . Choosing the Solvent; Potential Pitfalls solid must be sparingly soluble or insoluble at room temperature Not mixing it thoroughly - use a microspatula and rotate between fingers Being impatient and not giving it enough time to dissolve solid must be soluble at high temperature (boiling point of solvent) Not actually boiling the solvent Being impatient and not giving it enough time to dissolve Boiling for too long and evaporating much of the solvent nice crystals must form upon cooling Cooling too quickly and inducing precipitation - powder formation Not providing a nucleating surface for crystal formation - scratch the test tube walls with a glass stirring rod to create small glass particles Being impatient in step one and not recognizing that the compound was actually soluble at room temperature Adding too much solvent for the amount of solid used . . . . Choosing the Solvent: Hexanes 1 Petroleum ether 2 Ethyl acetate 3 1-Propanol 4 Ethanol (95%) 5 Distilled water 6 Unknown C solubility at room temperature No CE Unknown C solubility after heating Hs heyanes Pet the So 1- Unknown C solubility after cooling: Measuring out the unknown Unknown code: C -- 24 UN HU 1829 - . Dissolving the unknown Watch "Macroscale Recrystallization - Dissolution" Video on Blackboard - 39 seconds Pay particular attention to the volume of solvent added. The rubber bulbs on those pipettes hold about 1.5-2.0 ml when squeezed fully. Note that both solvent and solution are maintained at boiling temperature. Note the presence of a white boiling stone in the flask. It does not dissolve. Crystal Formation . . Watch "Macroscale Recrystallization - Crystal Formation" Video on Blackboard - 19 seconds Observe the timing - How long does it take from first visible crystals to a large amount? Note the size and structure of the crystals. To view the videos Click the video Click the two little arrows on the bottom right to view in full screen mode Click play To get back out of full screen, use the esc key on your keyboard . . Recrystallizing the Unknown: Potential Problems solid will not dissolve Grind up the material finely - large chunks will be harder to dissolve Mix it thoroughly - use a stirring rod, bust up any larger chunks Be patient - give it enough time to dissolve Add more solvent, a little at a time - make certain that both the contents of the Erlenmeyer flask and the pure solvent are boiling before adding more Use a boiling stone in both flasks to avoid "bumping and spillage nice crystals do not form upon cooling Don't mess with it while it cools to room temperature - you may induce precipitation Only place in an ice bath AFTER it reaches room temperature Provide a nucleating surface for crystal formation - scratch the test tube walls with a glass stirring rod to create small glass particles - you may have a supersaturated solution You added too much solvent - boil for a while to reduce solvent volume You chose the wrong solvent - see "Choosing the Solvent; Potential Pitfalls" . - ENTER Vacuum filtration set up: After vacuum filtration: APX-201 023 ZO Melting Point Technique Heat the apparatus quickly to within 25C of the mp (literature mp) and then reduce the rate of heating (to 2C/min) as you get close to the expected mp. Rapid heating near the mp leads to errors resulting from uneven heating through the sample and block. Mixed Melting Point (mmp) Mix the two compounds together to create a homogeneous sample Record the mp of this new sample . If the mmp gives a narrow range and matches the mp of each sample COMPOUNDS ARE IDENTICAL . If the mmp gives a broad range that is LOWER than the individual mp for each sample COMPOUNDS ARE DIFFERENT Recall that impure solids have broad mp ranges that are lower than literature values Identification of the unknown; Example . Mixing the compound with a different one decreases the m.p. a If one mixture has the same melting point as the unknown, it is evidence supporting the identity of the unknown. Example: (not the actual unknown you are using for this experiment!) Unknown mp 133-135C The unknown is mixed with: 1. Acetamide; mp of the mixture 76-80C 2. Urea; mp of the mixture 132-136C 3. Salicylic acid; mp of the mixture 112-114C The unknown can be concluded to be? Urea . . . mp (C) . OH 120-122 41-46 . Identification of the unknown: Our Experiment Table 2.2.List of possible Unknowns and Melting Point Data (www.SignaAldrich.com) Determine the melting point of the unknown. Compound mp (C) Compound Compare it to the mp of NH the compounds in the table to the right Mix the unknown with the two compounds with the OH closest mp. Determine the melting point of the two mixtures Judge which mixture shows the least sign of being impure. 113-115 47-51 121-124 . 112-115 134-138 HO for Q OH 134-136 Melting point observations: . . Recrystallized solid began melting at 134C, fully melted at 137C Which compounds from the table should we choose for the mixture mp? Recrystallized solid ground with acetylsalicylic acid and mixed melting point measured: began melting at 112C fully melted at 130C Recrystallized solid ground with benzoin and mixed melting point measured: began melting at 133C fully melted at 136C What can we conclude? . Experiment 2: Recrystallization of an Unknown-Macroscale Purpose The purpose of this experiment is to learn how to purify a larger sample of a crystalline compound through recrystallization. You will then gange your success by using the purity of your tecrystallized compound by measuring the melting point and identify the compound by and melting point . . Techniques Keeping a Lab Notebook Safety Pipets Cleaning and Drying Glassware Melting Points Recrystallization - . Assignments 1. Notebook preparation is required before lah. 2. Complete and submit before the assigned due date .. Online Pre-Lab Exercise: Recrystallization of an Unknown - Macroscale 3. Complete and submit by the start of the next lab period 2. Report Sheet: Recrystallization of an Unknown - Macroscale b. Online Post-Lab Questions Recrystallization of an Unknown - Macroscale Procedure Choosing the Solvent 1. Heat a 250-ml. beaker (or larger) of water on your hot plate. Add a couple of boiling stones. Check the temperature using a thermometer. You will want to initially heat the water to a temperature that is just above your LOWEST hosting solvent. 2. Obtain 1.3 g of an unknown sample. Record the code in your notebook Laban Recipale alipene Sol Cepat 22 3. Obtain 6 test tubes and label each with numbers 1-6. These number crepond to the went listed below Ines leche 2 lette 1-Prepanel thanol 5 Distilled water 4. Add approximately 0.10 g of your unknows to cach test tube and add 2 mL of the appropriate solvent. Shake the test tube to mix. If the solid dissolves at room temperature, put that sample aside, this solvent is NOT a good choice. If the solid does not dissolve at room temperature move on to step 5. Record this observation in your notebook 5. Take the samples still containing solid and suspend them in the hot water bath. Once the solvent inside the test tube is gently boiling, determine if the sold sample has disolved partially, completely, or not at all. If the solid has not dissolved at all remove this sample from the heat and set aside this solvent is NOT a good choice. Record this observation in your notebook 6. For the samples that are partially disolved, add additional solvent (you may wish to agitate the sample at this time) until the solid dissolves completely 7. Once your solid has completely dissolved with heating, remove the test tubes from the hot water bath and allow the lest tubes and contents to slowly cool to room temperature undisturbed. Do you see any crystal formation Record your observations 8. Once the test tube and contents have reached room temperature, carefully place the test tube in an ice bath for 10 min. Do you see any crystal formation Record your observatione 9. After the 10 min of cooling, examine the contents of the test tubes. Choose the solvent that resulted in the formation of the best crystals in the largest quantity. Record your solvent choice and reasoning in your notebook Recrystallization 10. Weigh the remaining solid unknown, record the weight in your notebook, and place all of the solid in a 50 mL Erlenmeyer flask. Add 1-2 bolling stones. Be sure to remove these latert 11. Place approximately 50 ml. of your chosen solvent in a 125-ml. Edenmeyer flask, wdd 1-2 boiling stones, and heat the solvent on your hot plate to a gentle boll Be careful to not heat the solvent foo hight You do not want the solvent to evaporale or ignite! 28 Recepten af impure Salle Constablety 12. Once the show is ingamell putin of the hot went to them. Firmy Hack with you holunni Parthen the plate small portion of bra, ing are the tank to the winntally down in the I went. It ha praticato in the latents of we do drugodt at the The idea is to relve your lil in a lot when the maintaining cometant volume. If your solent bookstovigortaly, the sevent will event and your lid will drive Remember. DISSOLVING is not the same 2 MELTING Y Joust want your sedal to melt or "oilout", leading to an impure sample. 13. Once the solid is completely divolved, remove the Erlenmeyer flask from the hot plate and allow it to cool SLOWLY to room temperature, undirbel 14. After you observe the formation of crystals, and the task has cooled all the way to room temperature cool the Erlenmeyer flank in an ice bath in order to maximize the amount of product that crystallines from the solution 15. Once you have cooled the Erlenmeyer flask in an ice bath for approximately 10 minutes isolate your crystals from the mother liquor by vacuum filtration. You should use a MINIMAL amount of ICE COLD recrystallizing solvent to transfer the remaining crystals . The crystals can then be washed with a MINIMAL amount of ICE COLD recrystallizing solvent. Room lemperature solvent will dissolve your crystals. 16. After allowing the crystals to dry, remove the boiling stones with forceps, measure the weight and record it in your notebook. Calculate the percent recovery and record it in your notebook 17. Obtain the melting point of your recrystallized and hopefully pure, unknown solid. Record the melting point range in your notebook 18. Compare your melting point to those listed in Table 32 of possible unknowns. Choose one compound from Table 3.2 and obtain a sample of the known compound from the reagents cart 19. You should now obtain a mixed melting point by grinding equal parts of your purified unknown and the known compound. A mortar and pestle is the best way to prepare the sample. Be sure to mix these compounds well! 20. Obtain the mixed melting point of the sample prepared in step 19. Record this value in your notebook. If the mixed melting point range is lower and broader than the melting point range for your pure compound, then the two compounds are not the same. If the mixed melting point range is identical (or nearly so) to that of your pure compound, then the two compounds are the same. 21. Using the mixed melting point data, correctly identify your unknown. Record this information 22. Place your purified sample in a glass vial, label it with your name, date, name of compound. and the measured melting point, and give it to your instructor In your notebook The Recitallaties ar lapure Salud Comprends Talle 2.2. Unawww Compound mpio Compound mp" NH OH 41-46 120 122 47.51 Ceo 121.124 NO 112-115 HO 114-130 113-115 134-136 Waste Disposal Any unused velvents should be placed in the CHEM 2254 waste contalter. All capillary tubes used for melting points should be placed in the broken glassware container. 30 Recitallisten af lepuresell Compensator Name Instructor Date Section Group Report Sheet: Laboratory 2b Recrystallization of Impure Solid Compounds: Experiment 2 PRE-LAB: The notebook pre-lah that you terned in prior to conducting the experiment DATA AND OBSERVATIONS Attach the carbon copy of your data and observations to the reportshert Part 1: Results 1. Unknown Code 2. Solvent choice for recrystallation z Fill in the following table and provide a brief statement for your choice of solvent in the space following the table See Sorrent tamilater (stroom temperature in terrains of pure Seile CINE 31 Percent recovery of un 4. Melting point of your pare unknown 3. Mixed melding point (mp) of your mixture Compound mixed with unknown Observed 6. Identity of your unknown (name and structure 32 Kristallation of impuresell Compounds Lantur Choosing the Solvent: solid must be sparingly soluble or insoluble at room temperature solid must be soluble at high temperature (boiling point of solvent) #1 & #3 dissolve at R7 1 #4& #5 NOT dissolve HOT. #2 dissolve HOT Unknown MINIMAL amount of solvent (barely cover the solid) . . Choosing the Solvent; Potential Pitfalls solid must be sparingly soluble or insoluble at room temperature Not mixing it thoroughly - use a microspatula and rotate between fingers Being impatient and not giving it enough time to dissolve solid must be soluble at high temperature (boiling point of solvent) Not actually boiling the solvent Being impatient and not giving it enough time to dissolve Boiling for too long and evaporating much of the solvent nice crystals must form upon cooling Cooling too quickly and inducing precipitation - powder formation Not providing a nucleating surface for crystal formation - scratch the test tube walls with a glass stirring rod to create small glass particles Being impatient in step one and not recognizing that the compound was actually soluble at room temperature Adding too much solvent for the amount of solid used . . . . Choosing the Solvent: Hexanes 1 Petroleum ether 2 Ethyl acetate 3 1-Propanol 4 Ethanol (95%) 5 Distilled water 6 Unknown C solubility at room temperature No CE Unknown C solubility after heating Hs heyanes Pet the So 1- Unknown C solubility after cooling: Measuring out the unknown Unknown code: C -- 24 UN HU 1829 - . Dissolving the unknown Watch "Macroscale Recrystallization - Dissolution" Video on Blackboard - 39 seconds Pay particular attention to the volume of solvent added. The rubber bulbs on those pipettes hold about 1.5-2.0 ml when squeezed fully. Note that both solvent and solution are maintained at boiling temperature. Note the presence of a white boiling stone in the flask. It does not dissolve. Crystal Formation . . Watch "Macroscale Recrystallization - Crystal Formation" Video on Blackboard - 19 seconds Observe the timing - How long does it take from first visible crystals to a large amount? Note the size and structure of the crystals. To view the videos Click the video Click the two little arrows on the bottom right to view in full screen mode Click play To get back out of full screen, use the esc key on your keyboard . . Recrystallizing the Unknown: Potential Problems solid will not dissolve Grind up the material finely - large chunks will be harder to dissolve Mix it thoroughly - use a stirring rod, bust up any larger chunks Be patient - give it enough time to dissolve Add more solvent, a little at a time - make certain that both the contents of the Erlenmeyer flask and the pure solvent are boiling before adding more Use a boiling stone in both flasks to avoid "bumping and spillage nice crystals do not form upon cooling Don't mess with it while it cools to room temperature - you may induce precipitation Only place in an ice bath AFTER it reaches room temperature Provide a nucleating surface for crystal formation - scratch the test tube walls with a glass stirring rod to create small glass particles - you may have a supersaturated solution You added too much solvent - boil for a while to reduce solvent volume You chose the wrong solvent - see "Choosing the Solvent; Potential Pitfalls" . - ENTER Vacuum filtration set up: After vacuum filtration: APX-201 023 ZO Melting Point Technique Heat the apparatus quickly to within 25C of the mp (literature mp) and then reduce the rate of heating (to 2C/min) as you get close to the expected mp. Rapid heating near the mp leads to errors resulting from uneven heating through the sample and block. Mixed Melting Point (mmp) Mix the two compounds together to create a homogeneous sample Record the mp of this new sample . If the mmp gives a narrow range and matches the mp of each sample COMPOUNDS ARE IDENTICAL . If the mmp gives a broad range that is LOWER than the individual mp for each sample COMPOUNDS ARE DIFFERENT Recall that impure solids have broad mp ranges that are lower than literature values Identification of the unknown; Example . Mixing the compound with a different one decreases the m.p. a If one mixture has the same melting point as the unknown, it is evidence supporting the identity of the unknown. Example: (not the actual unknown you are using for this experiment!) Unknown mp 133-135C The unknown is mixed with: 1. Acetamide; mp of the mixture 76-80C 2. Urea; mp of the mixture 132-136C 3. Salicylic acid; mp of the mixture 112-114C The unknown can be concluded to be? Urea . . . mp (C) . OH 120-122 41-46 . Identification of the unknown: Our Experiment Table 2.2.List of possible Unknowns and Melting Point Data (www.SignaAldrich.com) Determine the melting point of the unknown. Compound mp (C) Compound Compare it to the mp of NH the compounds in the table to the right Mix the unknown with the two compounds with the OH closest mp. Determine the melting point of the two mixtures Judge which mixture shows the least sign of being impure. 113-115 47-51 121-124 . 112-115 134-138 HO for Q OH 134-136 Melting point observations: . . Recrystallized solid began melting at 134C, fully melted at 137C Which compounds from the table should we choose for the mixture mp? Recrystallized solid ground with acetylsalicylic acid and mixed melting point measured: began melting at 112C fully melted at 130C Recrystallized solid ground with benzoin and mixed melting point measured: began melting at 133C fully melted at 136C What can we conclude