1. Rank the following compounds in order of increasing acidity. 2. Indicate which compounds would be more...
Question:
1. Rank the following compounds in order of increasing acidity.
2. Indicate which compounds would be more than 99% deprotonated by a solution of sodium ethoxide in ethanol.
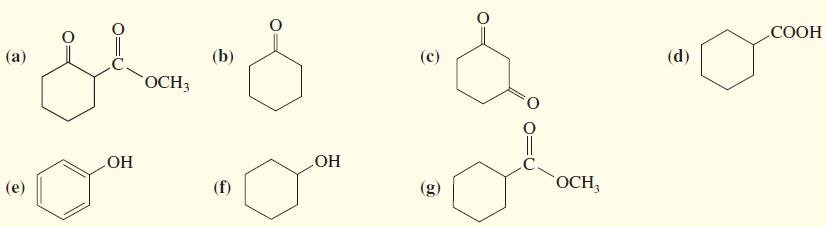
Transcribed Image Text:
СООН (а) (b) (с) (d) ОСН ОН ОН (е) (f) (g) OCH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
In order of increasing acidity The most acidic protons are shown in b...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Rank the following compounds in order of increasing acidity. Dont look at a table Of PKa data to help with your answer. (a) Benzoic acid, p-methyl benzoic acid, p-chlorohenzoic acid (b)...
-
Rank the following compounds in order of decreasing acidity:
-
Rank the following compounds in order of increasing stability based on relative ring strain.
-
For the attractive-nuisance doctrine to apply, the possessor need not be aware that children are likely to trespass on his land or have reason to know that the condition poses an unreasonable risk of...
-
Harwell Company manufactures automobile tires. On July 15, 2011, the company sold 1,000 tires to the Nixon Car Company for $50 each. The terms of the sale were 2/10, n/30. Harwell uses the net method...
-
What is the maximum amount of .12 debt (incremental) that RJR can support? What would be the effect of a higher interest rate?
-
A multiple comparison procedure for comparing four treatment means produced the confidence intervals shown here. Rank the means from smallest to largest. Which means are significantly different? 1m1...
-
Steelcase Inc. is one of the largest manufactures of office furniture in the United States. In Grand Rapids, Michigan, it produces filing cabinets in two departments: Fabrication and Trim Assembly....
-
These partially completed Income Statement columns from a 10-column work sheet are for the Winston Sailem Boat Rental Company for the year ended December 31, 2020. The owner's name is Carl Winston...
-
You have just been hired as a financial analyst for Lydex Company, a manufacturer of safety helmets. Your boss has asked you to perform a comprehensive analysis of the company's financial statements,...
-
Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with pyrrolidine.
-
Predict the products of the following aldol condensations. Show the products both before and after dehydration. (a) (b) (c) (d) (e) (f) CH3 TOH CH CH2-C-H CH TOH Ph-C-CH+ OH
-
What were the Catholic Church's views on marriage?
-
How do we design an electromagnetic sensor?
-
What is a virtual breadboard?
-
Joe secured a loan of $13,000 four years ago from a bank for use toward his college expenses. The bank charges interest at the rate of 9%/year compounded monthly on his loan. Now that he has...
-
Answer these two questions 1 32 2 Number of Units Sold 3 4 ! Direct Material units per unit of production 5 i 6 Total Direct Materials Used 7! 8 Price Per Unit 9 10 Cost of Direct Materials 11 12 13...
-
Give an algorithm for converting a tree to its mirror. Mirror of a tree is another tree with left and right children of all non-leaf nodes interchanged. The trees below are mirrors to each other....
-
Why do you think the managers and human resources department responded to this situation as they did?
-
Suppose that A is an m n matrix with linearly independent columns and the linear system LS(A, b) is consistent. Show that this system has a unique solution.
-
Starting with ketones and aldehydes of your choice, outline a directed aldol synthesis of each of the following using lithium enolates: (a) (b) (c) O OH CGH5 O OH
-
Assuming that dehydration occurs, write the structures of the two other products that might have resulted from the aldol cyclization just given. (One of these products will have a five-membered ring...
-
What starting compound would you use in an aldol cyclization to prepare each of the following? (a) (b) (c)
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...
-
Assume that an investment of $100,000 is expected to grow during the next year by 8% with SD 20%, and that the return is normally distributed. Whats the 5% VaR for the investment? A. $24,898 B....
-
Simpson Ltd is a small IT company, which has 2 million shares outstanding and a share price of $20 per share. The management of Simpson plans to increase debt and suggests it will generate $3 million...

Study smarter with the SolutionInn App


