10-Bromo--chamigrene, a compound isolated from marine algae, is thought to be biosynthesized from -bisabolene by the following...
Question:
10-Bromo-α-chamigrene, a compound isolated from marine algae, is thought to be biosynthesized from γ-bisabolene by the following route: Draw the structures of the intermediate bromonium and cyclic carbocation, and propose mechanisms for all three steps.
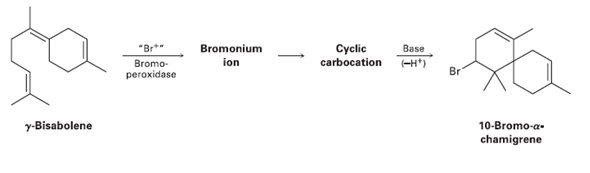
Transcribed Image Text:
Cyclic carbocation -H*) Bromonium "Br*" Bromo- Base ion Br peroxidase y-Bisabolene 10-Bromo-a- chamigrene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (12 reviews)
Br Br Bromo peroxid...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Isolated from marine algae, prelaureatin is thought to be biosynthesized from laurediol by the following route. Propose amechanism. . HO. "Br* Bromo- peroxidase Br- Laurediol Prelaureatin
-
A particular poultry disease is thought to be non communicable. To test this theory, 30,000 chickens were randomly partitioned into three groups of 10,000. One group had no contact with diseased...
-
The carbon monoxide in cigarettes is thought to be hazardous to the fetus of a pregnant woman who smokes. In a study of this hypothesis, blood was drawn from pregnant women before and after smoking a...
-
The 32-kg spool of outer radius r, = 420 mm has a centroidal radius of gyration k = 265 mm and a central shaft of radius r; = 155 mm. The spool is at rest on the incline when a tension T= 243 N is...
-
Waiting times (minutes) for a table at Joey's BBQ on Friday at 5:30 p.m. have quartiles Q1 = 21, Q2 = 27 and Q3 = 33. Using the inner fences as a criterion, would a wait time of 45 minutes be...
-
Find an SVD of the indicated matrix. -2 0
-
What information does the payback convey that is absent from the other capital budgeting decision methods? AppendixlLO1
-
Jen suspects that Brad is having an affair with Angie, and wants to divorce him. Her prenuptial agreement specifies that if she files for divorce, she receives $60,000, but that her award will double...
-
If a firm's sales change by 12% and it has a degree of operating leverage of 2.5 and a degree of financial leverage of 2.0. what is the expected change in earnings per share?
-
a. If Rashid chooses 3 books and 2 CDs, what is his marginal rate of substitution? b. If Rashid chooses 2 books and 6 CDs, what is his marginal rate of substitution? Rashid buys only books and CDs...
-
Iodine azide, IN 3 , adds to alkenes by an electrophilic mechanism similar to that of bromine. If a mono-substituted alkene such as 1-butene is used, only one product results: (a) Add lone-pair...
-
Draw the structure of a hydrocarbon that absorbs 2 molar equivalents of H2 on catalytic hydrogenation and gives only butane dial onozonolysis. H-CH2CH Butanedial
-
Evaluate log p 2 log 8 p.
-
In the circuit shown below, IE = 120 mA, a = 0.99, a2 = 0.98. Assuming that both transistors are in the active state, answer the following questions: 1. Find IC, IB, IE, IC, and Ic. 2. Find the...
-
Find the open intervals where the function is concave upward or concave downward. Find any inflection points. f(x)=-(x+1)6 Find any critical numbers for f and then use the second derivative test to...
-
Worked Example A two-dimensional incompressible flow is expressed by the velocity functions: u = -2xy y = y = x 1 w=0 Find the pressure field p(x, y) when the pressure at the origin (x=o, y=o) is p.....
-
The following information is available for Blue Spruce Corp. for 2022. Cash used to purchase treasury stock Cash dividends paid $111,592 50,576 Cash paid for interest Net income 51,968 1,077,176...
-
3 4pts Foam k MA C L H W F A box is transported by a truck. Suddenly, the driver uses the brakes and applies 100 N deceleration force to the box. The weight of the box is 20 Kg and its dimensions are...
-
Consider the duopoly computer industry problem with heterogeneous consumers analyzed in Section 2.2.3, but suppose that each computerproducing firm bears a positive production cost. More precisely,...
-
Suppose that a flow network G = (V, E) violates the assumption that the network contains a path s t for all vertices V. Let u be a vertex for which there is no path s u t. Show that there must...
-
Explain why water drops are spherical in the absence of gravity.
-
What rate law would be expected for the reaction of cyanide ion -:CN) with ethyl bromide b: the SN2 mechanism?
-
Give the structure of the alkyl halide product expected (if any) in each of the following reactions. (a) HOCH2CH2CH2OH + excess HI (b) heat 25 "C (CH3)sCCH OH HCI (Hint: pee Fig. 9.4. p. 392.)
-
Draw both the complete structure and the abbreviated structure, and give another name for each of the following compounds. (a) methl p - toluenesulfonate (b) cyclohexyl mesylate
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...
-
Regarding Enron, this was a company that resulted in the creation of the Sarbanes-Oxley Act and many reforms to the accounting profession. Research the company and answer the following...

Study smarter with the SolutionInn App


