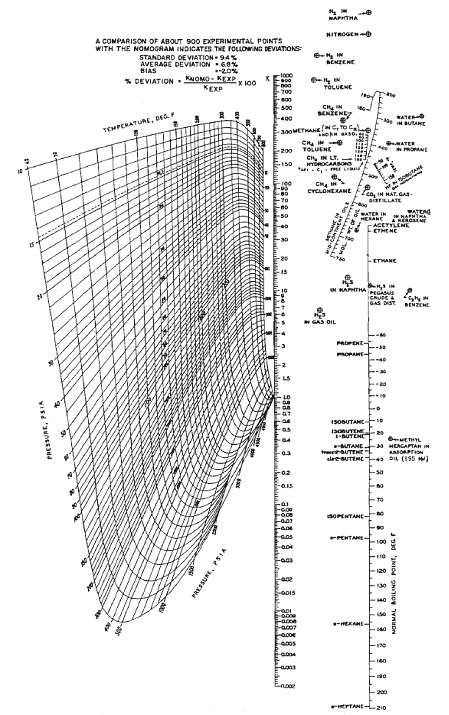
Question: (a) Find the bubble-point temperature of the following mixture at 50 psia, using K-values from figure. (b) Find the temperature that results in 25% vaporization
(a) Find the bubble-point temperature of the following mixture at 50 psia, using K-values from figure.
(b) Find the temperature that results in 25% vaporization at this pressure. Determine the corresponding liquid and vaporcompositions.
NAPHTHA NITROGEN A COMPARISON OF ABOUT 900 EXPERIMENTAL POINTS WITH THE NOMOGRAM INDICAT ES THE FOLLOWNG DEVIATIONS STANDARD OEVIATION- 94% AVERAGE DEVATION 6.% BENZEHE BIAS -1000 * DEVIATION . KNOMO- KEXP X 100 TOLUENE KEXP -700 -800 CH N SENENEY 500 RATE -400 TEMPERATURc, eF -300METHANENG, TOC ATER TOLUENE 200 CH, IN LT. mROCAABOa -ree CYCLONEXANE Lea.IN HAT.GA A MATER IN HERANE GME ACEYYLENE CTHENC *RAPH ETHANE MATHAA IN BENZEHE GAS DIL PROPENE PROPANE- 1SOBUTANE IaonuTEE -BUTENE UTANE teaUTEHt eleesUTEME DL (155 20 - HEACAPTAN IN AASORPTION -02 -sa -0.15 -ai Fo.ce sOPENTANE- PEHTANE- 70,00- T-acce -a007 HEKAME- 140 200 EPTANEL210 wietui.LILJ PRKSSURE. PSIA
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
Instead of using Figs 28 and 29 for Kvalues use SRK method with the CHEMCAD simulator with the follo... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (128).docx
120 KBs Word File


