Question: A liquid mixture of 27 wt% acetone and 73 wt% water is to be separated at 25 o C into a raffinate and extract by
A liquid mixture of 27 wt% acetone and 73 wt% water is to be separated at 25oC into a raffinate and extract by multistage, steady-state, countercurrent liquid-liquid extraction with a solvent of pure 1, 1, 2-trichloroethane. Phase equilibrium data are given in Exercise 8.11. Determine:
(a) The minimum solvent-to-feed ratio to obtain a raffinate that is essentially free of acetone.
(b) The composition of extract at the minimum solvent-to-feed ratio.
(c) The composition of the extract stream leaving stage 2 (see Figure), if a large number of equilibrium stages is used with the minimum solventrate.
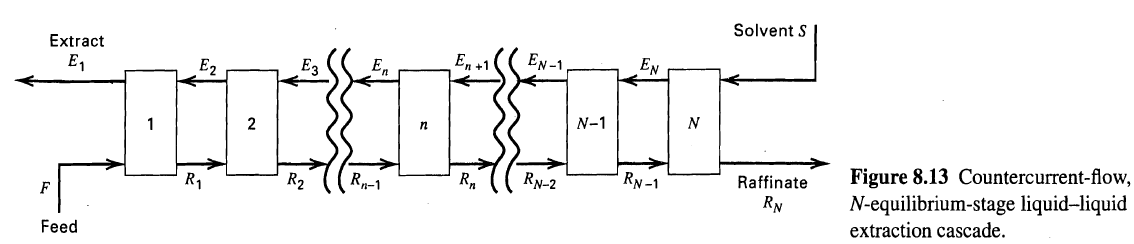
Solvent S Extract EN-1 EN En +1 En E, E2 N-1 Figure 8.13 Countercurrent-flow, N-equilibrium-stage liquid-liquid extraction cascade. RN-1 Raffinate R, Rp-1 R2 R1 RN-2 RN Feed
Step by Step Solution
3.52 Rating (169 Votes )
There are 3 Steps involved in it
Subject Extraction of acetone A from water C by 1 1 2trichloroethane S at 25 o C Given Liquid mixture of 27 wt A and 73 wt C Liquidliquid equilibrium ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (322).docx
120 KBs Word File


