Question: A natural gas containing 82.0 mole% CH4 and the balance C2H6 is burned with 20% excess air in a boiler furnace. The fuel gas enters
A natural gas containing 82.0 mole% CH4 and the balance C2H6 is burned with 20% excess air in a boiler furnace. The fuel gas enters the furnace at 298 K, and the air is preheated to 423 K. The heat capacities of the stack gas components may be assumed to have the following constant values:
(a) Assuming complete combustion of the fuel, calculate the adiabatic flame temperature.
(b) How would the flame temperature change if the percent excess air were increased? How would it change if the percentage of methane in the fuel increased? Briefly explain both of youranswers.
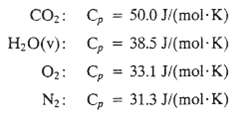
CO2: HO(v): C, = 50.0 J/(mol-K) C = 38.5 J/(mol-K) O: Cp 33.1 J/(mol-K) N: Cp 31.3 J/(mol-K)
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
a Basis 100 mol natural gas 82 mol CH4 18 mol CH6 CH4 g 20 g COg 2HOv A89036 kJmol CH6g Og 2COg 3HOv ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (568).docx
120 KBs Word File


