Question: (a) Show that for a reversible heat pump the energy required per unit of heat delivered inside the building is given by the Carnot efficiency
(a) Show that for a reversible heat pump the energy required per unit of heat delivered inside the building is given by the Carnot efficiency (6):
W/Qh = ηc = (τh – τt)/τh
What happens if the heat pump is not reversible?
(b) Assume that the electricity consumed by a reversible heat pump must itself be generated by a Carnot engine operating between the temperatures τhh and τl. What is the ratio Qhh/Qh, of the heat consumed at τhh, to the heat delivered at τh? Give numerical values for Thh = 600K; Th = 300K; Tl = 270k.
(c) Draw am emergy-entropy flow diagram for the combination heat pump, similar to figures, but involving no external work at all. Only energy and entropy flows at three temperatures.
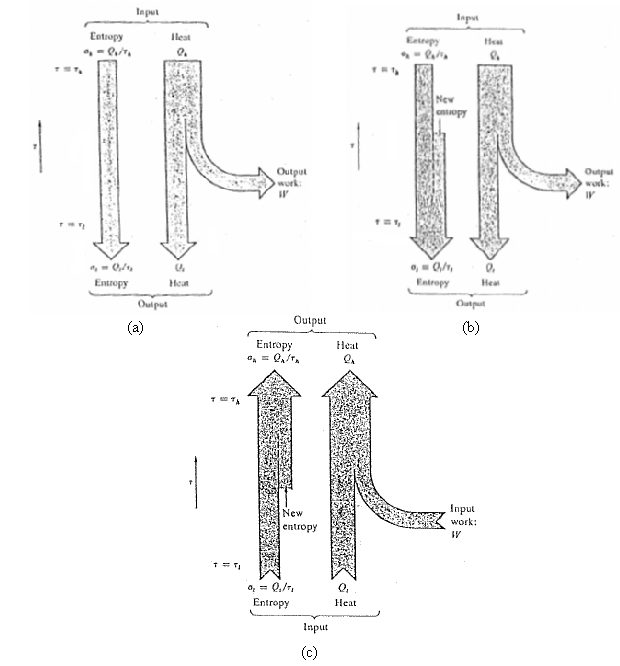
Input p Entropy Ilest Eetrepy He New entopy Output work: Outpul work: IV 0,0/, E Ouiput Entropy Output Output (b) (a) Entropy Heat Input work: New entropy a, = Q,/, Entropy Heat Input (c)
Step by Step Solution
3.32 Rating (170 Votes )
There are 3 Steps involved in it
a If operating reversibly this is just a carnot engine with al... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
42-P-S-S-T-T (81).docx
120 KBs Word File


