A All-cis Cyclodecapentaene is a stable molecule that shows a single absorption in its 1H NMR spectrum
Question:
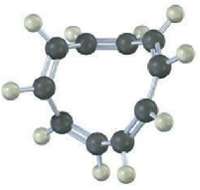
A All-cis Cyclodecapentaene is a stable molecule that shows a single absorption in its 1H NMR spectrum at 5.67 ?. Tell whether it is aromatic, and explain its NMR spectrum.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
The allcis Cyclodecapentaene shown here is not aromatic be...View the full answer

Answered By

Muhammad Rehan
Enjoy testing and can find bugs easily and help improve the product quality.
4.70+
10+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
[18]-Annulene shows two signals in its 1H NMR spectrum: one at 9.25 ppm and the other very far upfield (beyond TMS) at - 2.88 ppm. What hydrogens are responsible for each of the signals? . . ...
-
In a 1H NMR spectrum the peak from TMS is found to occur at 500.134 271 MHz. Two other peaks in the spectrum are found at 500.135 021 and 500.137 921 MHz; compute the chemical shifts of these two...
-
1 H NMR spectrum E corresponds to a molecule with the formula C 4 H 8 O. Its IR spectrum has important bands at 945, 1015, 1665, 3095, and 3360 cm -1 . (a) Determine the structure of the unknown. (b)...
-
Presented below is information related to Larkspur Inc.'s inventory, assuming Larkspur uses lower-of-LIFO cost-or-market. (per unit) Skis Boots Parkas Historical cost $224.20 $125.08 $62.54 Selling...
-
What are some of the ways companies can continue to motivate their employees in a time of a recession and extended higher unemployment.
-
FIGURE EX30.14 shows a 10-cm-diameter loop in three different magnetic fields. The loop??s resistance is 0.20 . For each, what are the size and direction of the induced current? a. b. c. X X
-
Secure a copy of an express warranty, and analyze it for differences with an implied warranty. AppendixLO1
-
The Dynaco Manufacturing Company has an assembly line that feeds two drill presses. As partially completed products come off the line, they are lined up to be worked on as drill presses become...
-
QS 4-4 (Algo) Computing net invoice amounts LO P1 Compute the amount to be paid for each of the four separate invoices assuming that all invoices are paid within the discount period
-
An online store sells a product for which the daily demand is normally distributed with a mean of 120 units and with a standard deviation of 10 units. We assume the source of supply has a constant...
-
Give IUPAC names for the following substances (red = O, blue =N): (a) (b)
-
A 1, 6-Methanonaphthalene has an interesting 1 H NMR spectrum in which the eight hydrogen?s around the perimeter absorb at 6.9 to 7.3 ?, while the two CH 2 protons absorb at ?0.5 ?. ?Tell whether it...
-
What are the different applications of DFS and BFS?
-
Conservation efforts include reintroduction of species into the wild from captive breeding programs. Leung et al. (2018) rewilded mice from the inbred laboratory strain of mouse, C57BL/6, that had...
-
The ending balance of the Accounts Receivable account was \(\$ 7,800\). Services billed to customers for the period were \(\$ 21,500\), and collections on account from customers were \(\$ 23,600\)....
-
Cash Flow Activity Classification Classify each activity as financing, investing, or operating: 1. Repay a loan from a bank. 2. Sell merchandise from a storefront operation. 3. Dispose of an old...
-
Generally Accepted Accounting Principles Select the best answer to each of the following MBC) questions: 1. Accounting rules are developed to provide: a. Simplicity b. Useful information c....
-
Basic Accounting Principles Identify whether the following statements are true or false. 1. Together the revenue recognition principle and the expense recognition (matching) principle define the...
-
understand the basic notion of business partnerships;
-
Gordon and Lisa estimate that they will need $1,875,000 in 40 years for their retirement years. If they can earn 8 percent annually on their funds, how much do they need to save annually?
-
Consider the reaction between iodine gas and chlorine gas to form iodine monochloride: A reaction mixture at 298 K initially contains P I2 = 0.25 atm and P Cl2 = 0.25 atm. What is the partial...
-
Glucose is the most abundant monosaccharide. From memory, draw glucose in (a) The Fischer projection of the open chain (b) The most stable chair conformation of the most stable pyranose anomer (c)...
-
Without referring to the chapter, draw the chair conformations of (a) -D-mannopyranose (the C2 epimer of glucose) (b) -D-allopyranose (the C3 epimer of glucose) (c) -D-galactopyranose (the C4 epimer...
-
Use Figure 23-3 (the D family of aldoses) to name the following aldoses. (a) The C2 epimer of D-arabinose (b) The C3 epimer of D-mannose (c) The C3 epimer of D-threose (d) The enantiomer of...
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


