All of the stereo isomers of 1, 2, 3, 4, 5, 6-hexachlorocyclohexane have very similar rates of
Question:
All of the stereo isomers of 1, 2, 3, 4, 5, 6-hexachlorocyclohexane have very similar rates of E2 reaction except the following stereo isomer, which reacts about 7000 times more slowly than the others.Explain.
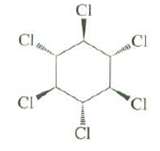
Transcribed Image Text:
CI Cl .CI 'CI CI CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
In the stereo isomer of 1 2 3 4 5 6hexachlorocyclohexane shown the Cl atoms ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Determine CPI, ETC (1), and EAC. Activity Total PV 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1 6 6 2 20 10 10 3 30 9 6 6 6 3 4 20 8 2 5 5 5 16 4 4 4 4 6 18 9 9 7 8 4 4 Status Report: Ending Period 5 Task %...
-
(1) Given A and i. (1a) Find the equivalent amount at the end of year 1. (1b) Find the equilvalent amount at the end of year B. (2) Given X and i, Find the annual equivalent A from year S to 8. *...
-
Macmillan Learning A Geiger-Muller tube is a type gas-filled radiation detector. It can detect particles like X-rays, alpha particles, and beta rays (electrons). This is useful in quantizing the...
-
Explain the meaning of the following terms in the schedule of cost of goods manufactured: (a) Total manufacturing costs. (b) Manufacturing costs to account for. (c) Cost of goods manufactured.
-
The following table shows the effects of five transactions (1 through 5) on the assets, liabilities, and equity of Mulan's Boutique. Identify the explanation from a through j below that best...
-
List various methods for improving project communications, such as running effective meetings, using various technologies effectively, and using templates? LO.1
-
At the beginning of the school year, Priscilla Wescott decided to prepare a cash budget for the months of September, October, November, and December. The budget must plan for enough cash on December...
-
a. Verify the July 16, 2013, asked yield of 0.69 percent on the Treasury bond, stripped principal STRIP maturing August 2016. Use a two-day settlement period from the date of purchase (i.e.,...
-
Devin Wolf Company has the following balances in selected accounts on December 31, 2017. Devin has a calendar year end. Accounts Receivable.............................................$0 Accumulated...
-
Show the products of this reaction. How would the composition of the products change if t-BuO ? in t-BuOH were used in place of ethoxide ion in ethanol? ELOH + CH,CH,0
-
Show the product of thisreaction: Ph Br- - + NaOEt Br EIOH Ph
-
The edge roughness of slit paper products increases as knife blades wear. Only 1% of products slit with new blades have rough edges, 3% of products slit with blades of average sharpness exhibit...
-
Financial Statement Items Identify the financial statement (or statements) in which each of the following items would appear: income statement (IS), statement of stockholders' equity (SSE), balance...
-
Recall from Chapter 4 that Tiger Stripe Copy Center is a small business located near a large university campus. Tiger Stripe Copy offers a range of services to walk-in customers, including passport...
-
Accounting Processes Identify the following processes as either measuring or communicating. a. Prepare financial statements for the entity b. Identify relevant economic activities of the entity c....
-
To estimate future values of the cost indices, one is tempted to assume that the average value for the year occurred at midyear (June 30-July 1) and that the linear fit to the recent data can be...
-
Reston Manufacturing Corporation produces a cosmetic product in three consecutive processes. The costs of Department | for May 2016 were as follows: Department | handled the following units during...
-
What are the situations where differences exist in the accounting treatment recommended in the Indian Accounting Standards (A.S.) and the International Accounting Standards (I.A.S.)?
-
Define the term utility software and give two examples.
-
Solve each system by graphing. y=-x+1 y=x-3 -4 -2 + 3 12 y 1 et 2 byht for LA X
-
The following 1H NMR absorptions were obtained on a spectrometer operating at 300MHz. Convert the chemical shifts from units to hertz downfield from TMS. (a) 2.1 (b) 3.45 (c) 6.30 (d) 7.70
-
When measured on a spectrometer operating at 200 MHz, chloroform (CHCl3) shows a single sharp absorption at 7.3 . (a) How many parts per million downfield from TMS does chloroform absorb? (b) How...
-
How many signals would you expect each of the following molecules to have in its 1H and 13Cspectra? (c) CH -H (b) () CH CHCH CH C CH (f) (e) (d) "T CH "
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


