Amino acids can be prepared (as their conjugate bases) by the reaction of a bromine substituted carboxylic
Question:
Amino acids can be prepared (as their conjugate bases) by the reaction of a bromine substituted carboxylic acid with excess ammonia.
(a) The conjugate base of the amino acid alanine is formed in the following reaction. Show the structure of the product and explain why an excess of ammonia is required. Explain which mechanism the reaction follows.
(b) Show how a similar reaction could be used to prepare the conjugate base of the amino acidphenylalanine.
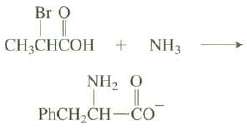
Transcribed Image Text:
Br O CH,CHCOH + NH3 NH, 0 PHCH,CH-CO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 47% (17 reviews)
a An excess of ammonia is required because the first ammonia r...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show how crossed Claisen condensations could be used to prepare the following esters. (a) (b) (c) (d) Ph C-CH-C OCH,CH CH CH-C-OCH3 C-C-oCH Eto C CH-C OCH,CH Ph CH,CH,CH,
-
Show how ethyl alcohol could be used to prepare (a) CH3CN and (b) CH3CH2CN. Along with ethyl alcohol you may use any necessary inorganic reagents.
-
Show how ethyl alcohol could be used to prepare (a) CH3CN and (b) CH3CH2CN. Along with ethyl alcohol you may use any necessary inorganic reagents.
-
I need one or more queries on the basis of database of netflix. I can upload the picture of database Create Index Modify Table Schema Delete Table Print CREATE TABLE Dates ( [PK] INTEGER PRIMARY KEY...
-
How common are product costing systems in practice? Why might a business choose to do without a product costing system?
-
Pro-Form acquired 70 percent of Clip-Rite on June 30, 2017, for $910,000 in cash. Based on Clip-Rite's acquisition-date fair value, an unrecorded intangible of $400,000 was recognized and is being...
-
Demonstrate an understanding of the major difference between purchase and pooling of interests accounting. AppendixLO1
-
Candlewood LLC started business on September 1, 2016 and adopted a calendar year. During 2016, Candlewood incurred $6,500 in legal fees for drafting the LLC's operating agreement and $3,000 in...
-
A corporate bond has a coupon rate of 11% (paid semiannually) and matures on November 15, 2025. Its quoted price is 94. Assume 30 days per month. It is now July 15, 2015. What is the invoice (or...
-
A large firm has 85% of its service calls made by a contractor, and 10 of these calls result in customer complaints. The other 15% of the service calls are made by their own employees, and these...
-
The two nitrogen of the following dipeptide have very different re-activities as nucleophiles explain which nitrogen is the betternucleophile. : : NH,CH,CNHCH,COCH,
-
Explain which of the two acetate esters, product 1 or product 2, is formed when the alkyl chloride is reacted with sodium acetate in DMSO. Problems using online three-dimensional molecular models
-
Finders Investigative Services is an investigative services firm that is owned and operated by Stacy Tanner. On June 30, 2019, the end of the fiscal year, the accountant for Finders Investigative...
-
On October 1, Deloitte \& Coopers Price started a consulting firm. The asset, liability, and stockholders' equity account balances after each of the firm's first six transactions are shown below....
-
On June 1, a group of bush pilots in British Columbia, Canada, formed the Adventure Airlines, Inc., by selling \(\$ 51,000\) of common stock for cash. The group then leased several aircraft and...
-
During the first year of operation, 2011, Martin's Appliance recognized \$292,000 of service revenue on account. At the end of 2011 , the accounts receivable balance was \(\$ 57,400\). Even though...
-
During May, Willett Corp. purchased direct materials for 4,250 units at a total cost of \($61,625\). Willetts standard direct materials cost is \($14\) per unit. Prepare the journal entry to record...
-
Determine a positive real root of this equation using appropriate software: \[ 3.5 x^{3}-10 x^{0.5}-3 x=-4 \]
-
What ethical values or principles are involved in the lack of accurate reporting of cracks at the nuclear reactors in the late 1980s and 1990s?
-
An environmentalist wants to determine if the median amount of potassium (mg/L) in rainwater in Lincoln County, Nebraska, is different from that in the rainwater in Clarendon County, South Carolina....
-
What is the ratio of number of movies Bob saw to the number that Emily saw? Number of Movies Seen in Theaters in 1 year Bob Emily John Alice BAAD = 4 Movies
-
Which has higher energy, infrared radiation with = 1.0 x 10 6 m or an X ray with = 3.0 x 10 9 m? Radiation with v = 4.0 x 10 9 Hz or with = 9.0 x 10 6 m?
-
Its useful to develop a feeling for the amounts of energy that correspond to different parts of the electromagnetic spectrum. Calculate the energies of each of the following kinds of radiation (a) A...
-
What functional groups might the following molecules contain? (a) A compound with a strong absorption at 1710 cm1 (b) A compound with a strong absorption at 1540 cm1 (c) A compound with strong...
-
Ellis Perry is an electronics components manufacturer. Information about the company's two products follows: \ table [ [ , , , ] , [ Units produced,AM - 2 , FM - 9 , ] , [ Direct labor hours required...
-
Which of the following requirements to claim Earned Income Tax Credit is TRUE? The credit can be claimed under any filing status. The taxpayer must have a valid SSN for employment in the U.S., issued...
-
Olde Tyme Beverage Companys operating activities for the year are listed below. Cost of Goods Manufactured $131,000 Operating expenses 80,000 Beginning inventory, FG 16,000 Ending inventory, FG...

Study smarter with the SolutionInn App


