Question: An enthalpy-concentration diagram is given in Figure for a mixture of n-hcxanc (H). and n-octane (0) at 101 kPa. Using this diagram, determine the following:
An enthalpy-concentration diagram is given in Figure for a mixture of n-hcxanc (H). and n-octane (0) at 101 kPa. Using this diagram, determine the following:
(a) The mole-fraction composition of the vapor when a liquid containing 30 mol% H is heated from point A to the bubble-point temperature at point B.
(b) The energy required to vaporize 60 mol% of a mixture initially at l00oF and containing 20 mol% H (point G).
(c) The compositions of the equilibrium vapor and liquid resulting from part(b).
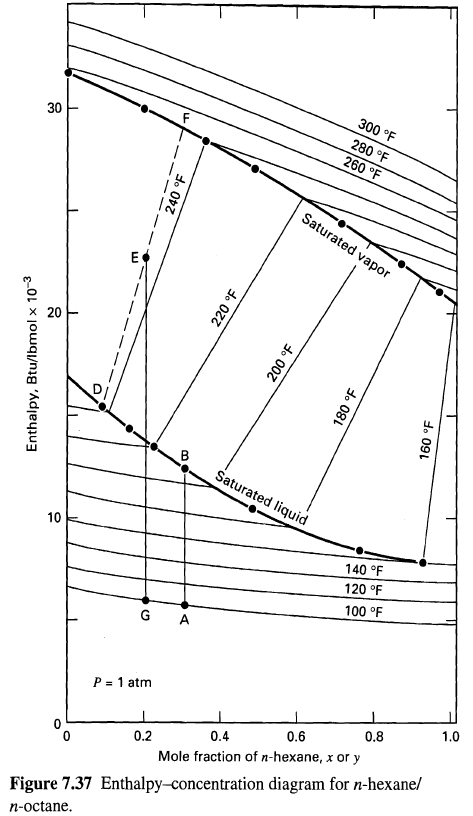
300 F 280 F 260 F 30 Saturated vapor 20 Saturated liquid 10 140 F 120 F 100 F G A P = 1 atm 1.0 0.8 0.6 0.4 0.2 Mole fraction of n-hexane, x or y Figure 7.37 Enthalpy-concentration diagram for n-hexane/ n-octane. Enthalpy, Btu/lbmol x 10-3 240 F 220 F B. 200 F do 081 do 091
Step by Step Solution
3.43 Rating (169 Votes )
There are 3 Steps involved in it
a In Fig below the bubble point for 30 mol nC 6 in nC 8 is shown as point B which corresponds to a t... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (296).docx
120 KBs Word File


