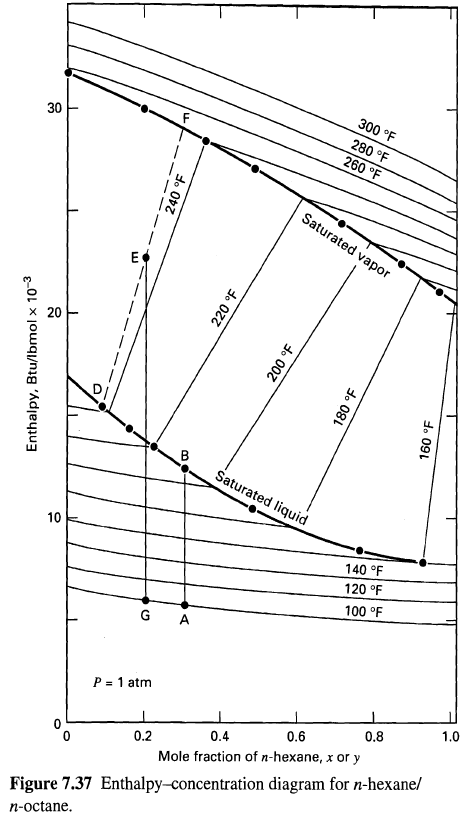
Using the enthalpy'concentration diagram of Figure, determine the following for a mixture of n-hexane (H) and n-octane
Question:
Using the enthalpy'concentration diagram of Figure, determine the following for a mixture of n-hexane (H) and n-octane (0) at I atm:
(a) The temperature and compositions of equilibrium liquid and vapor resulting from adiabatic mixing of 950 lb/h of a mixture of 30 mol% H in O at l8oF with 1,125 Ib/h of a mixture of 80 mol% H in O at 24oF.
(b) The energy required to partially condense, by cooling, a mixture of 60 mol% H in O from an initial temperature of 26oF to 200oF. What are the compositions and amounts of the resulting vapor and liquid phases per pound-mole of original mixture?
(c) If the equilibrium vapor from part (b) is further cooled to 180oF, determine the compositions and relative amounts of the resulting vapor andliquid.
Step by Step Answer:






