Question: An equimolar mixture of ethane, propane, n-butane, and n-pentane is subjected to a flash vaporization at 150?F and 205 psia. What are the expected amounts
An equimolar mixture of ethane, propane, n-butane, and n-pentane is subjected to a flash vaporization at 150?F and 205 psia. What are the expected amounts and compositions of the liquid and vapor products? Is it possible to recover 70% of the ethane in the vapor by a single-stage flash at other conditions without losing more than 5% of nC4 to the vapor? Obtain K-values fromFigure.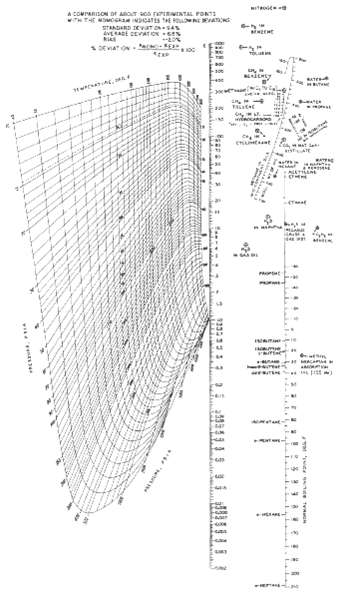
A COMARISON Dr Aor acs rIENINTAL PORT1 STAADRD IEVar SAS TALate ALLTILINE
Step by Step Solution
3.48 Rating (171 Votes )
There are 3 Steps involved in it
Take as a basis a feed of 100 lbmolh From Fig at 150 o F and 205 psia the Kvalues are as given in the table below Then solve the RachfordRice equation ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (113).docx
120 KBs Word File


