Question: An SO2-air mixture is being scrubbed with water in a countercurrent-flow packed tower operating at 20C and 1 atm. Solute-free water enters the top of
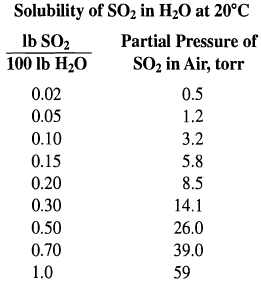
An SO2-air mixture is being scrubbed with water in a countercurrent-flow packed tower operating at 20°C and 1 atm. Solute-free water enters the top of the tower at a constant rate of 1,000 lb/h and is well distributed over the packing. The liquor leaving contains 0.6 lb S02/100 lb of solute-free water. The partial pressure of SO2 in the spent gas leaving the top of the tower is 23 ton. The mole ratio of water to air is 25. The necessary - equilibrium data are given below.
(a) What percent of the SO2 in the entering gases is absorbed in the tower?
(b) In operating the tower it was found that the rate coefficients kp and kL remained substantially constant throughout the tower at the following values:

At a point in the tower where the liquid concentration is 0.001 lbmol SO2 per lbmol of water, what is the liquid concentration at the gas-liquid interface in lbmol/ft3? Assume that the solution has the same density asH20.
kL = 1.3 ft/h k, = 0.195 lbmol/h-ft-atm kp
Step by Step Solution
3.35 Rating (167 Votes )
There are 3 Steps involved in it
a Inlet water rate 10001802 555 lbmolh Inlet air rate water rate25 55525 222 lbmolh Partial pressure of air in exit gas 760 23 737 torr By partial pre... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (230).docx
120 KBs Word File


