Question: Analysis of a capillary flow meter (see Fig. 2B.8). Determine the rate of flow (in lb/hr) through the capillary flow meter shown in the figure.
Analysis of a capillary flow meter (see Fig. 2B.8). Determine the rate of flow (in lb/hr) through the capillary flow meter shown in the figure. The fluid flowing in the inclined tube is water at 20oC, and the manometer fluid is carbon tetrachloride (CC14) with density 1.594 g/cm3. The capillary diameter is 0.010 in. Note: Measurements of H and L are sufficient to calculate the flow rate; ? need not be measured. Why?
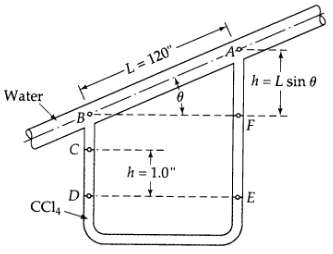
Water -Bo Co -L=120". Do CC14- 17 h = 1.0" A h= L sin 0 IF E
Step by Step Solution
3.51 Rating (175 Votes )
There are 3 Steps involved in it
Designate the water by fluid I and the carbon tetrachlorid... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
6-E-C-E-T-P (27).docx
120 KBs Word File


