Aromatic sulfonation can be reversed by hearting an arylsulfonic acid in steam: (a) Draw a curved-arrow mechanism
Question:
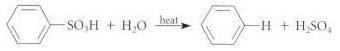
(a) Draw a curved-arrow mechanism for this transformation.
(b) Explain why compound C cannot be synthesized in one step from thiophene.
Transcribed Image Text:
-н + н.so,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (13 reviews)
a By the principle of microscopic reversibility the mechanism for th...View the full answer

Answered By

Aun Ali
I am an Associate Member of Cost and Management Accountants of Pakistan with vast experience in the field of accounting and finance, including more than 17 years of teaching experience at university level. I have been teaching at both undergraduate and post graduate levels. My area of specialization is cost and management accounting but I have taught various subjects related to accounting and finance.
5.00+
13+ Reviews
32+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Treating oxytocin with certain reducing agents (e.g., sodium in liquid ammonia) brings about a single chemical change that can be reversed by air oxidation. What chemical changes are involved?
-
Monomethylation of histone Arg residues can be reversed by the action of a peptidylarginine deiminase, which requires water to remove the methyl group along with the imino group of Arg. Draw the...
-
Can a radical be aromatic?
-
Lewis and Associates has been in the termite inspection and treatment business for five years. The following is a list of accounts for Lewis on June 30, 2017. It reflects the recurring transactions...
-
a. Identify the main determinants for valuation of feature films, television programs, and general release feature productions by Columbia Pictures. b. Are the bases of valuation reasonable? Explain....
-
When the gross profit statements option is chosen, a separate report is generated for each department. (True/False)
-
Evaluation of flexography printing plates. Refer to the Journal of Graphic Engineering and Design (Vol. 3, 2012) study of the quality of flexography printing, Exercise 10.34 (p. 552). Recall that...
-
Cocoaheaven processes cocoa beans into cocoa powder at a processing cost of $9,500 per batch. Cocoaheaven can sell the cocoa powder as is or it can process the cocoa powder further into either...
-
Orange Corp. uses the indirect method to prepare its statement of cash flows. Refer to the following information for the year: 1. Long-Term Notes Payable, beginning balance, $80,000 2. Long-Term...
-
A convenience store is considering changing its layout to encourage impulse buying. The triangular flow matrix below gives the measure of association between different product groups ( e. g., beer,...
-
The dipole moments of pyrrole and pynolidine are similar in magnitude but have opposite directions. Explain, indicating the direction of the dipole moment in each compound. Should the dipole moment...
-
Provide curved-arrow mechanisms for each of the reactions given in Fig. P25.41. Give the structure for the intermediates A and B in parts (d) (a) (b) + DSo, (dilute) + HCI
-
Why is measuring performance important to an organization?
-
According to a recent study, 21% of American college students graduate with no student loan debt. Suppose we obtain a random sample of 106 American college students and record whether or not they...
-
Differentiate the following with respect to x: a. y=5x+2x + x + 15 b. y=4x+3x - 4x - 10 c. y = 3Sin(5x) d. y = 3Cos(3x) e. y=10e -25x f. y = log(6x)
-
Question 2. The rate of drug destruction by the kidneys is proportional to the amount of the drug in the body. The constant of proportionality is denoted by K. At time t the quantity of the drug in...
-
5. 6. -1 (4a) U u X2 1 X2 -2 x -1 -2 12 (4b) U -2 2 Y y 16 x2 X2 3 1 (4c) U u - x 2 Y y -8 Y y -20 5 x X2 2 Find the state space models of the three systems shown in Fig. 4a, Fig. 4b, and Fig. 4c,...
-
Given the following data for Mehring Company, compute total manufacturing costs, prepare a cost of goods manufactured statement, and compute cost of goods sold. Direct materials used $230,000...
-
Why is Jensens alpha computationally efficient?
-
On August 31, 2012, the balances of the accounts appearing in the ledger of Wood Interiors Company, a furniture wholesaler, are as follows:Prepare the August 31, 2012, closing entries for Wood...
-
How would you prepare the following ethers using a Williamson synthesis? (a) Methyl propyl ether (b) Anisole (methyl phenyl ether) (c) Benzyl isopropyl ether (d) Ethyl 2, 2-dimethylpropyl ether
-
Review the mechanism of oxymercuration shown in figure, and then write the mechanism of the alkoxy mercuration reaction of 1-methylcyclopentene with ethanol. Use curved arrows to show the electron...
-
How would you prepare the following ethers? Use whichever method you think is more appropriate, the Williamson synthesis or the alkoxy mercuration reaction. (a) Butyl cyclohexyl ether (b) Benzyl...
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...
-
Lime Corporation, with E & P of $500,000, distributes land (worth $300,000, adjusted basis of $350,000) to Harry, its sole shareholder. The land is subject to a liability of $120,000, which Harry...
-
A comic store began operations in 2018 and, although it is incorporated as a limited liability company, it decided to be taxed as a corporation. In its first year, the comic store broke even. In...

Study smarter with the SolutionInn App


