Question: Assign structures to compounds with the following 1H NMR spectra:? (a) C 4 H 7 ClO? ??IR: 1810 cm ?1 ? (b) C 5 H
Assign structures to compounds with the following 1H NMR spectra:?
(a) C4H7ClO? ??IR: 1810 cm?1?
(b) C5H7NO2? ?IR: 2250, 1735 cm?1?
(c) C5H10O2? ? ?IR: 1735 cm?1?
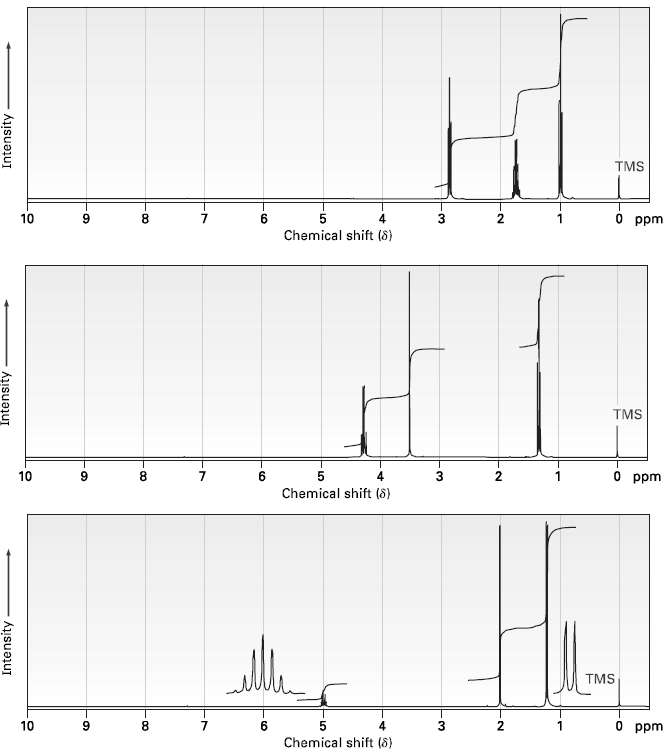
TMS O ppm 10 8. 6. Chemical shift (8) TMS O ppm 10 8. 2 Chemical shift (8) TMS O ppm 10 8. 6. Chemical shift (8) Intensity Intensity Intensity -00 - LO
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
a CH3CHCHCCI a b c a 1... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-CA (166).docx
120 KBs Word File


