Assume that the magnetic moment of an aluminum atom is 1 Bohr magneton. The density of aluminum
Question:
Assume that the magnetic moment of an aluminum atom is 1 Bohr magneton. The density of aluminum is 2.7 g/cm3, and its molecular mass is 27 g/mol.
(a) Calculate Ms and ?0Ms for aluminum.
(b) Use the results of Problem 71 to calculate ?m at T = 300 K.
(c) Explain why the result for part (b) is larger than the value listed in Table 29-1.
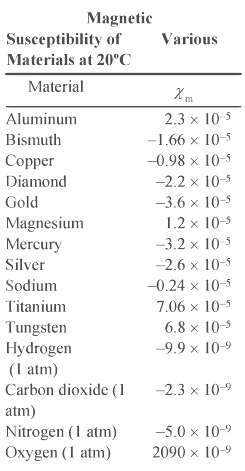
Transcribed Image Text:
Magnetic Susceptibility of Materials at 20C Various Material X m 2.3 x 10 5 Aluminum -1.66 x 10-5 Bismuth Copper Diamond -0.98 x 10-5 -2.2 x 10-5 Gold -3.6 x 10-5 1.2 x 10-5 -3.2 x 10-5 Magnesium Mercury Silver -2.6 x 10-5 Sodium -0.24 x 10-5 Titanium 7.06 x 10-5 Tungsten Hydrogen (I atm) 6.8 x 10-5 -9.9 x 10-9 Carbon dioxide (1 -2.3 x 10-9 atm) Nitrogen (1 atm) -5.0 x 10-9 Oxygen (1 atm) 2090 x 10-9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a M s N A M B M s 602 10 28 927 10 24 Am M s 558 10 ...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Electricity and Magnetism questions
-
Calculate the magnitude of the spin magnetic moment of an electron.
-
The magnetic moment of the Earth is approximately 8.00 x 1022 A (m2. (a) If this were caused by the complete magnetization of a huge iron deposit, how many unpaired electrons would this correspond...
-
Calculate the magnetic moment of an atom (in Bohr magnetons) (a) In 1F state; (b) In 2D3/2 state; (c) In the state in which S = 1, L = 2, and Lande factor g = 4/3.
-
Compare to traditional organizational development approaches. To get back on track and find a way to function more smoothly, should this taskforce use an Appreciative Inquiry approach or a more...
-
On March 31, 2019, the Federal Unemployment Tax Payable account in the general ledger of The Argosy Company showed a balance of $1,507. This represents the FUTA tax owed for the first quarter of the...
-
While eating his Kelloggs Frosted Flakes one January morning, Tony noticed the following article in his local paper: Kellogg Company Reports Fourth-Quarter 2017 Results and Provides Guidance For 2018...
-
Prepare fund financial statements for the general fund. AppendixLO1
-
Ricks English Hut (Ricks) is a restaurant located in North Myrtle Beach, South Carolina on a saltwater marsh, surrounded by stately oak trees. Ricks appetizers and entrees run the gamut, from tasty...
-
Ceteris paribus, the longer the term to maturity of a bond, the _____ the bonds price risk. Group of answer choices more less same all of the above three choices are possible
-
1. What business research problem does Royal Barton face? What are his information needs? Outline some survey research objectives for a research project on the Royal Bee system. 2. What type of...
-
In a simple model of paramagnetism, we can consider that some fraction f of the molecules have their magnetic moments aligned with the external magnetic field and that the rest of the molecules are...
-
A toroid with N turns carrying a current I has mean radius R and cross-sectional radius r, where r R
-
NRG Energy plans to construct a giant solar plant in Santa Teresa, New Mexico, to supply electricity to West Texas Electric. The plant will have 390,000 heliostats to concentrate sunlight onto 32...
-
Fineas Co. use the Job Order Costing system to determine product costs. Before entering 2020, the company has created a production budget, with an estimated total manufacturing overhead of $...
-
Define what a market value is? What are three major principles of investing funds? How does the federal government control the money supply? An investor purchases a 10-year U.S. Treasury note and...
-
1. Suppose we have two alternative designs, each of which yields a different present value of the total lifetime cost: the first is $1604 and the second is $1595. Verify that the present value of the...
-
Sometimes when we are asked for a linear model, the information that we are given is data about a scenario. In these cases we have to use Excel to generate a trendline. There is a video in this...
-
1. Purpose Explain 3 points from the Introduction section as to why this study is important. How did this study build on the existing literature in this area? 2. Participants Outline at least 2...
-
Does Applegate Construction Company produce a product or provide a service? Would the buyer of a typical subdivision house answer this question differently than a customhouse buyer? lop5
-
According to a recent survey, 40% of millennials (those born in the 1980s or 1990s) view themselves more as spenders than savers. The survey also reveals that 75% of millennials view social...
-
A company wants 800 square feet of carpet, but the carpet store sells only by the square meter. How many square meters does the company need to buy? (1 m = 39.37 in.)
-
Write the Van der Waals equation via the reduced parameters , , and T, having taken the corresponding critical values for the units of pressure, volume, and temperature. Using the equation...
-
Knowing the Van der Waals constants, find: (a) The maximum volume which water of mass m = 1.00 kg can occupy in liquid state; (b) The maximum pressure of the saturated water vapour.
-
Calculate the temperature and density of carbon dioxide in critical state, assuming the gas to be a Van der Waals one.
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


