Question: Benzene (B) and chlorobenzene (C) are being separated in a distillation column. Vapor and liquid streams, each containing both species, are fed to one of
Benzene (B) and chlorobenzene (C) are being separated in a distillation column. Vapor and liquid streams, each containing both species, are fed to one of the trays of the column, and liquid and vapor streams are taken off the tray. The tray functions as an ideal stage (see Problem 6.63): the effluent streams are in equilibrium at temperature T and pressure P. with compositions related by Raoult?s law, Equation 6.4-1.
Calculate the number of degrees of freedom. Then specify sets of design variables for which the solution for the remaining state variables would be?
(a) Straightforward,?
(b) Iterative, and?
(c) Impossible.
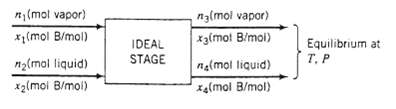
(mol vapor) x(mol B/mol) n(mal liquid) x(mol B/mol) IDEAL STAGE n3(mol vapor) x3(mot B/mol) (mol liquid) x4(mol B/mol) Equilibrium at T. P
Step by Step Solution
3.46 Rating (166 Votes )
There are 3 Steps involved in it
10 variables N3 N4 X X X3 X4 T P 2 material balances 2 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (576).docx
120 KBs Word File


