Calculate the potential energy of attraction between the Na + and Cl ? ions at the equilibrium
Question:
Calculate the potential energy of attraction between the Na+ and Cl? ions at the equilibrium separation r0 = 0.236 nm and compare this result with the dissociation energy given in Figure. What is the energy due to repulsion of the ions at the equilibrium separation?
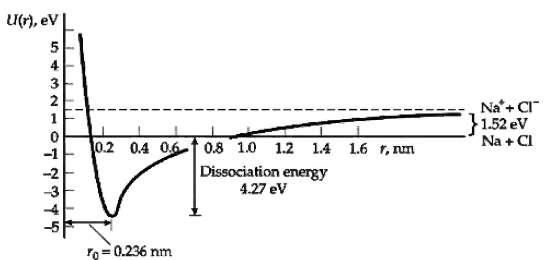
Transcribed Image Text:
U(r), ev 3 2 Na'+ CI" }1.52 ev Na + CI 0.2 0.4 06-f 0.8 1.0 1.2 14 1.6 1, nm Dissociation energy 4.27 ev = 0.236 nm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)
U e ke 2 r U e 1440236 eV 610 eV Dissociation ene...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Modern Physics questions
-
FIGURE Q25.2 shows the potential energy of a proton (q = +e) and a lead nucleus (q = +82e). The horizontal scale is in units of femtometers, where 1 fm = 10 -15 m. a. A proton is fired toward a lead...
-
The potential energy of a diatomic molecule (a two-atom system like H2 or O) is given by where r is the separation of the two atoms of the molecule and A and B are positive constants. This potential...
-
The potential energy of a particle constrained to move along the x -axis is shown in the graph. At x = 0, the particle is moving in the + x -direction with a kinetic energy of 400 J. Can this...
-
1.1. Define a want and a need, and give an example of each. Then, explain how the relationship between wants and needs affects the way people spend money. (4 points) 2.. Identify and describe an...
-
Fabio Company uses a voucher system. Record the following transactions in the voucher register: 201X June 8 Purchased office equipment on account from Tiani Corporation, $1,200; voucher no. 300 was...
-
Maryanne paid $300 for a call option on 100 shares of a stock. The option gives her the right to buy the stock for $27 per share until March 1. On February 15, the stock price rises to $32 per share,...
-
54. Tall Tree LLC was recently formed with the following members: Name Tax Year- End Capital/Profits % Eddie Robinson December 31 40% Pitcher Lenders LLC June 30 25 Perry Homes Inc. October 31 35...
-
Silver Lining, Inc., provides investment advisory services. The company adjusts its accounts monthly, but performs closing entries annually on December 31. The firms unadjusted trial balance dated...
-
Font Paragraph Styles When is this style appropriate (Ctrl) COMPROMISING (Half Way) Intermediate in both assertiveness and cooperativeness. Compromises are not optimal solutions. Give-and-take...
-
Richard chooses technique 0 and 2 requiring 10+10-20 efforts and provising 10+11=21 benefits. Hence, 21 is returned as the output Example 2: input1: 3 input2: (10,10,10,10) input3: (10,11,12,15)...
-
Indicate the mean value of r for two vibration levels in the potential-energy curve for a diatomic molecule and show that because of the asymmetry in the curve, rav increases with increasing...
-
The equilibrium separation of the K + and F ions in KF is about 0.217 nm. (a) Calculate the potential energy of attraction of the ions, assuming them to be point charges at this separation. (b) The...
-
Definitions. Define the terms in the key terms list.
-
1. What gives stainless steels their good corrosion resistant properties? 2. Which stainless steel is the lowest cost and why? 3. What are some characteristics of Nickel Alloys? 4. What are the 2...
-
Problem 4. Determine the motion of a two-dimensional linear oscillator of potential energy V = kr
-
5 Informatics solutions in the "complex and catastrophic" end of the population-risk spectrum must support which type of services/functions? 1 point Intensive case management Wellness program
-
What are the characteristics of products that Otis Trains produces? What are order qualifiers and winners? Explain at least three advantages and three drawbacks of offshoring to JLPTC. What risks are...
-
Find the angle and length of the resulting vector for the given d and e vectors by the analytical method. After that, find the parameters of the resulting vector for the three vectors. In the answer,...
-
How will you organize employees on the farm, and how will you assign responsibility to Smith and Jones? You can recommend any assignment that you like, but the numbers of employees that are required...
-
Choose two matrices A and B with dimension 2 x 2. Calculate det A, det B, and det (AB). Repeat this process until you are able to discover how these three determinants are related. Summarize your...
-
_______ contraction drives blood through the systemic and pulmonary circuits; outside the heart, blood pressure is highest in the _______. a. Atrial; ventricles b. Atrial; atria c. Ventricular;...
-
In attempting to discern distant details, people will sometimes squint. Why does this help?
-
Is the image formed on the retina of the human eye upright or inverted? Discuss the implications of this for our perception of objects.
-
The human eye is much like a camera- yet, when a camera shutter is left open and the camera is moved, the image will be blurred; but when you move your head with your eyes open, you still see...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated
-
Vaughn Company sells two types of pumps. One is large and is for commercial use. The other is smaller and is used in residential swimming pools. The following inventory data is available for the...

Study smarter with the SolutionInn App


