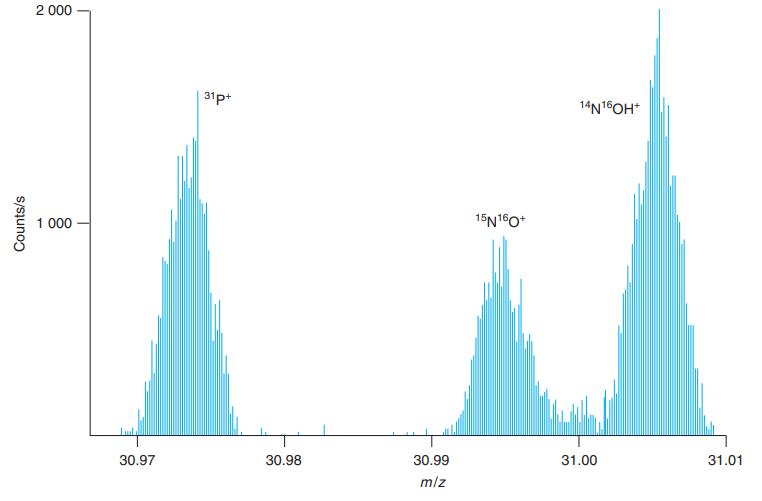
Calculate the theoretical masses of the species in Figure 21-9 and compare your answers with the values
Question:
Calculate the theoretical masses of the species in Figure 21-9 and compare your answers with the values observed in the figure.
Figure 21-9
Transcribed Image Text:
2 000 31p+ 14N16OH* 1 000 15N160* 30.97 30.98 30.99 31.00 31.01 m/z Counts/s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
31P 31P e 30973 760000 55 30973 21 observed 309735 To measure mz I enlar...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Calculate the theoretical air-fuel ratio on a mass and mole basis for the combustion of ethanol, C2H5OH.
-
Calculate the theoretical air-fuel ratio on a mass and mole basis for the combustion of ethanol, C2H5OH.
-
Calculate the theoretical density of -Sn. Assume the diamond cubic structure and obtain the atomic radius information from Appendix B.
-
What implications might the difference in initial training provision between the USA and Germany have for the organisation of work and the character of relationships between managers and...
-
Terando Co. began operations on July 1. It uses a perpetual inventory system. During July, the company had the following purchases and sales. Instructions (a) Determine the ending inventory under a...
-
The consumer purchase of specific brands is an indication of the relationship that develops over time between a company and its customers. Locate and retrieve the most current ranking of best global...
-
What are some advantages of having a crossfunctional team develop the business case? AppendixLO1
-
Manufacturing Firm Kildeer Company makes easels for artists. During the last calendar year, a total of 30,000 easels were made, and 31,000 were sold for $52 each. The actual unit cost is as follows:...
-
can you do a summary of this Studies to purchase Study 8 - Segment Media Preferences Study 9 - Market Forecast Study 14 - Product Attribute Perceptions Study 15 - Attribute Importance by Segement...
-
Data collected from selected major metropolitan areas in the eastern United States show that 2% of individuals living within the city limits move to the suburbs during a one-year period while 1% of...
-
The mass of a fragment ion in a high-resolution spectrum is 83.086 5 Da. Which composition, C 5 H 7 O + or C6H + 1 1, better atches the observed mass?
-
Consider the extraction of Mn+ from aqueous solution into organic solution by reaction with protonated ligand, HL: Rewrite Equation 22-13 in terms of Kextraction and express Kextraction in terms of...
-
List the types of approaches used in recommendation engines.
-
Spitfire Company makes and sells three products: A, B, and C. The following data relate to these products: A B Demand in units Selling price per unit 110 100 90 $180 $210 $195 Raw material costs per...
-
NCF & Partners (NCF) is a firm of CPAslocated in Whitby that has been in business for 20 years. NCF's revenue has declined steadily over the past few years. The partners are looking for ways...
-
Task 4.2Written report Describe how you will present the menu to customers, for example, folders, covers, boards or binding. Include details of colour schemes, pictures, icons, logos, symbols and...
-
The American company "Amazonian", leader in food distribution, is starting operations in Brazil. They just hired a group of new managers who will lead several branches of the company in different...
-
1; Assume you are in charge of fundraising for an organization on your campusa social fraternity or sorority, a business fraternity, or any other such organization. It is your job to identify a...
-
Why does water have a lower vapor pressure at 25 C than dimethyl ether (CH 3 OCH 3 )? Which of these two liquids has the greater enthalpy of vaporization?
-
Illini Company, Inc. Balance Sheet as of 12/31/20X0 Assets Current Assets: Cash $1,500,000 Accounts receivable, net 18,000 Inventory 50,000 Total current assets 1,568,000 Equipment 90,000 Goodwill...
-
A 50.0-mL solution containing Ni 2+ and Zn 2+ was treated with 25.0 mL of 0.045 2 M EDTA to bind all the metal. The excess unreacted EDTA required 12.4 mL of 0.012 3 M Mg 2+ for complete reaction. An...
-
Considering just acid-base chemistry, not ion pairing and not activity coefficients, use the systematic treatment of equilibrium to find the pH and concentrations of species in 1.00 L of solution...
-
What is the difference between E and E o for a redox reaction? Which one runs down to 0 when the complete cell comes to equilibrium?
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


