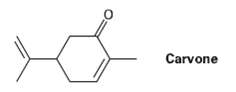
Carvone is the major constituent of spearmint oil. What products would you expect from reaction of carvone
Question:
Carvone is the major constituent of spearmint oil. What products would you expect from reaction of carvone with the following reagents?
(a) (CH3)2Cu ? Li +, then H3O +?
(b) LiAlH4, then H3O
(c) CH3NH2?
(d) C6H5MgBr, then H3O+
(e) H2/Pd?
(f) CrO3, H3O+
(g) (C6H5)3P+C?HCH3?
(h) HOCH2CH2OH, HCI
Transcribed Image Text:
Carvone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
a d g O HO ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What products would you expect from reaction of 1-methylcyclohexene with the following reagents? (a) Aqueous acidic KMnO4 (b) O3, followed by Zn, CH3CO2H
-
What products would you expect from reaction of the following alkenes with NBS? If more than one product is formed, show the structures ofall. H (a) . (b) CH3D2H
-
What products would you expect from reaction of estradiol (Problem 27.44) with the following reagents? (a) NaH, then CH3I (b) CH3COCI, pyridine (c) Br2, FeBr3 (d) Pyridinium chlorochromate in CH2Cl2
-
What are the side effects of the drugs that are used for treatment of Multiple Sclerosis attacks? Are cataracts a result of steroid use? Is osteoporosis a complication of Multiple Sclerosis?
-
How does Goodwill use controls to make informed decisions about its resources?
-
Ruxton, Wilkinson, and Neuhuser (2015) stated that researchers will frequently be required to consider whether a sample of data appears to have been drawn from a normal distribution. Based on the...
-
distinguish between main research approaches: deductive and inductive. LO6
-
Refer to the Amazon.com financial statements, including Notes 1 and 3, in Appendix A at the end of this book. Answer the following questions. Requirements 1. Which depreciation method does Amazon use...
-
Q3. Option Implied volatility at Different Strike, aka vol skew (10 points). You observe the following price for VXX options: VXX = 25.50. Expiration in 3 months. Calls Put Last Price Change % Change...
-
The State of Dakota seeks to increase the investment of new business in the state by providing the best wireless communications environment in the country. Dakota has vast land areas suitable for...
-
How would you synthesize the following substances from benzaldehyde and any other reagentsneeded? (c) (b) (a) CH- CH2
-
The SN2 reaction of (dihromomethyl) benzene, C6H5CHBr2, with NaOH yields benzaldehyde rather than (dihydroxymethyl) benzene, C6H5CH (0H)2 Explain.
-
A wheel is rolling on a straight level track with a uniform velocity ' \(v\) '. The instantaneous velocity of a point on the wheel lying at the mid-point of a radius. (a). varies between \(3...
-
Show how you would go about balancing the following equations: Cu + HNO3 Cu(NO3)2 + NO + H2O HIO3 + Fel2 + HCI FeCl3 + ICI + H2O 2.Conservation of mass A student places 0.58 g of iron and 1.600 g...
-
Sales MOSS COMPANY Income Statement For Year Ended December 31, 2021 Cost of goods sold Gross profit Operating expenses (excluding depreciation) Depreciation expense Income before taxes Income taxes...
-
Prior to the Covid-19 epidemic, Master's and Ph.D. programs in psychology required applying students to submit their scores on the standardized graduate admission exam (GRE). For the past three...
-
Benicio wants to make sure that the Sales table does not contain any duplicate records, which would make any sales analysis incorrect. Identify and remove duplicate records in the Sales table as...
-
University Car Wash purchased new soap dispensing equipment that cost $261,000 including installation. The company estimates that the equipment will have a residual value of $27,000. University Car...
-
Teams and Work Groups Working Across Networks Synopsis The skills of working across boundaries that are increasingly required for successful team work are also important in networks, and other loose...
-
Consider the sections of two circuits illustrated above. Select True or False for all statements.After connecting a and b to a battery, the voltage across R1 always equals the voltage across R2.Rcd...
-
The formal charges on the O atoms in the ion [ONO] + is (a) -2; (b) -1; (c) 0; (d) +1.
-
In the presence of sodium ethoxide the following transformation occurs. Explain. (1) NaOEt (2) HCI OEt OEt
-
Thymine is one of the heterocyclic bases found in DNA. Starting with ethyl propanoate and using any other needed reagents, show how you might synthesize thymine. CH3 Thymine
-
Predict the products from each of the following aldol reactions. (a) (b) (c) (d) (e) H NaOH H20 H H NaOH H2O o'H H HOEN NaOH H2O H NaOH H2O
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...
-
An investor wants to purchase a zero coupon bond from Timberlake Industries today. The bond will mature in exactly 5.00 years with a redemption value of $1,000. The investor wants a 12.00% annual...

Study smarter with the SolutionInn App


