Question: Certain solid substances, known as hydrated compounds, have well-defined molecular ratios of water to some other species, which often is a slat. For example, calcium
Certain solid substances, known as hydrated compounds, have well-defined molecular ratios of water to some other species, which often is a slat. For example, calcium sulfate dehydrate (commonly known as gypsum, CaSO4 ? 2H2O), has 2 moles of water per mole of calcium sulfate; alternatively, it may be said that 1 mole of gypsum consists of 1 mole of calcium sulfate and 2 moles of water. The water in such substances is called water of hydration. Solid gypsum is formed in a crystallizer and leaves that unit as a slurry (a suspension of solid particles in a liquid) of solid gypsum particles suspended in an aqueous CaSO4 solution. The slurry flows from the crystallizer to a filter in which the particles are collected as a filter cake. The filter cake, which is 95.0 wt% solid gypsum and the remainder CaSO4 solution, is fed to a dryer in which all water (including the water of hydration in the crystals) is driven off to yield anhydrous (water-free) CaSO4 as product. A flowchart and relevant process data are given below.
Solids content of slurry leaving crystallizer: 0.35 kg CaSO4 ? 2H2O/L slurry
CaSO4 content of slurry liquid: 0.209g CaSO4/100g H2O
Specific gravities: CaSO4 ? 2H2O(s) 2.32; liquid solution, 1.05
(a) Briefly explain in your own words the fu8nctions of the three units (crystallizer, filter, and dryer)
(b) Take a basis of one liter of solution leaving the crystallizer and calculate the mass (kg) and volume (L) of solid gypsum, the mass of CaSO4 in the gypsum, and the mass of CaSO4 in the liquid solution.
(c) Calculate the percentage recovery of CaSO4-that is, the percentage of the total CaSO4 (precipitated plus dissolved) leaving the crystallizer recovered as solid anhydrous CaSO4.
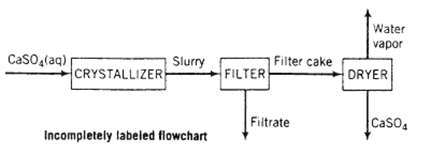
CaSO4(aq) CRYSTALLIZER Slurry Incompletely labeled flowchart FILTER Filter cake Filtrate Water vapor DRYER CaSO4
Step by Step Solution
3.46 Rating (175 Votes )
There are 3 Steps involved in it
a Unit Crystallizer Filter Dryer b m V gypsum gypsum Functi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (65).pdf
180 KBs PDF File
13-E-C-E-C-P (65).docx
120 KBs Word File


