Circle the conjugated pi bonds, if any, in the following compounds? c) CH,CH=CH-C=N b) CH3-CH=CH-C-CH3 d) H-C=C-CH=CH-C-H
Question:
Circle the conjugated pi bonds, if any, in the following compounds?
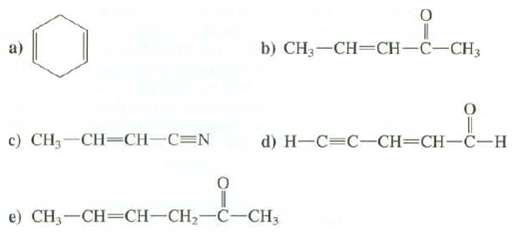
Transcribed Image Text:
c) CH,—CH=CH-C=N b) CH3-CH=CH-C-CH3 d) H-C=C-CH=CH-C-H La e) CH₂-CH=CH-CH₂-C-CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
a The bonds are not conjugated because they are ...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Using numerical locants and the names in Table 11.1 as a guide, give an acceptable IUPAC name for each of the following compounds
-
Circle each compound that has a conjugated Ï system:
-
In Theorem 3.37, prove that if p i = p j , then we cannot determine whether an error occurs in the ith or the jth component of the received vector. (Throughout this proof we denote by ai the ith...
-
To determine the focal length of a lens, you place the lens in front of a small lightbulb and then adjust a viewing screen to get a sharply focused image. Varying the lens position produces the...
-
Reliable Cleaning services was started a number of years ago by Pick Elber to provide cleaning services to both large and small businesses in his home city. Over the years, as local businesses...
-
A company uses the percent of sales method to determine its bad debts expense. At the end of the current year, the company's unadjusted trial balance reported the following selected amounts: All...
-
A process is under control and follows a normal distribution with mean 100 and standard deviation 10. In constructing a standard x@chart for this process, the control limits are set 3 standard...
-
Consider the data set shown in Table 7.8. The first attribute is continuous, while the remaining two attributes are asymmetric binary. A rule is considered to be strong if its support exceeds 15% and...
-
Goodwill is the excess of the purchase price of the acquired enterprise over the book value of the identifiable net assets acquired. fair value of the identifiable net assets acquired. book value of...
-
Consumer Reports provided overall customer satisfaction scores for AT&T, Sprint, T-Mobile, and Verizon cell-phone services in major metropolitan areas throughout the United States. The rating for...
-
Show the hybridization at each of the atoms, except H, in these molecules. Indicate the type of each designated bond and the orbital's that are overlapping to form it. : a) H_C_C,H 4 (both) : ...
-
What is the hybridization at the indicated atoms in these compounds? a) CHCH=CH_NHCH3 12 3 c) CH,=CH0CH, 1 2 3 4 5 0: b) CHC0CH, 1 d) 2 1 NH
-
Haddad Corporation operates a retail computer store. To improve delivery services to customers, the company purchases four new trucks on April 1, 2015. The terms of acquisition for each truck are...
-
You want to retire after working 35 years with savings in excess of $1,100,000. You expect to save $3,300 a year for 35 years and earn an annual rate of Interest of 11%. (Round your answer to 2...
-
FOLLOW ALL INSTRUCTIONS AND GENERATE YOUR CODE AFTER READING THE JUNIT TESTS, THAT IS ALL THE METHODS AND CONSTRUCTORS YOU USE SHOULD BE BASED ON THE JUNIT TESTS PROVIDED. I HAVE ATTATCHED THE JAVA...
-
Are some values in the class data grossly different from all the others? If so, check for errors in calculation or procedure that would allow to objectively eliminate the data. 2. Do the range values...
-
An aging analysis of Uli Limited's accounts receivable at December 3 1 , 2 0 2 4 and 2 0 2 3 , showed the following: Number of Days Outstanding Accounts Receivable Estimated Percentage Uncollectible...
-
(Linear momentum) Two jets of liquid, one with specific gravity 1.00 and the other with specific gravity 1.33, collide and form one homogeneous jet as shown in the figure below. Determine (a) the...
-
Describe what the physical situation would look like if you were responsible for creating a non-threatening environment.
-
D Which of the following is considered part of the Controlling activity of managerial accounting? O Choosing to purchase raw materials from one supplier versus another O Choosing the allocation base...
-
Determine whether the series converges or diverges. In n n=1 n
-
Identify the functional groups in each of the following molecules: (a) Methionine, an amino acid: (b) Ibuprofen, a pain reliever: (c) Capsaicin, the pungent substance in chilipeppers: ||...
-
Propose structures for simple molecules that contain the following functional groups: (a) Alcohol (b) Aromatic ring (c) Carboxylic acid (d) Amine (e) Both ketone and amine (f) Two double bonds
-
Identify the functional groups in the following model of arecoline, a veterinary drug used to control worms in animals. Convert the drawing into a line-bond structure and a molecular formula (red =...
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


