Question: Coal containing 5.0 wt% S is burned at a rate of 1250lb m /mm in a boiler furnace. All of the sulfur in the coal
Coal containing 5.0 wt% S is burned at a rate of 1250lbm/mm in a boiler furnace. All of the sulfur in the coal is oxidized to SO2. The product gas is sent to a scrubber in which most of the SO2 is removed, and the scrubbed gas then passes out of a stack. An Environmental Protection Agency regulation requires that the gas in (he stack must contain no more than 0.018lbm S02/lbm coal burned. To test compliance with this regulation a flow meter and an SO2 analyzer are mounted in the stack. The volumetric flow rate of the scrubbed gas is found to be 2867 ft3/s, and the SO2 analyzer reading is 37. Calibration data for the analyzer are given in the table below.
(a) Determine the equation that relates SO2 concentration in lbm/ft3 to the analyzer reading.
(b) Is the process in compliance with the EPA regulation?
(c) What percentage of the SO2 produced in the furnace is removed in the scrubber?
(d) An earlier EPA regulation set a limit on the mole fraction of SO2 in the gas emerging from the stack (as opposed to the quantity of SO2 emitted per mass of coal burned), but a way was found to release large quantities of SO2 from stacks without violating this regulation. Speculate on what the method of getting around the old regulation was.?
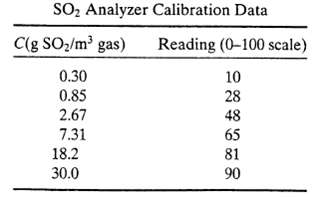
SO Analyzer Calibration Data C(g SO/m gas) 0.30 0.85 2.67 7.31 18.2 30.0 Reading (0-100 scale) 10 28 48 65 81 90
Step by Step Solution
3.38 Rating (179 Votes )
There are 3 Steps involved in it
a b c Plot C log scale vs R linear scale on semilog paper get straight line through R 10 C 030 gm an... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (144).pdf
180 KBs PDF File
13-E-C-E-C-P (144).docx
120 KBs Word File


