Complete the following reactions. OH NO2 Cr(VI) HSO4
Question:
Complete the following reactions.
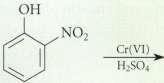
Transcribed Image Text:
OH NO2 Cr(VI) HSO4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
A paraquinon...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Complete the following nuclear reactions: (c) 1 (o, p)
-
Complete the following nuclear reactions and find their Q values (use Appendix V for masses if necessary): (a) Li +_ (b) U + in (c) Be(a, %C He + He 5(dn) + Sr
-
Complete the following nuclear reactions. a. b. c. ISP + ? Si 14
-
Suppose the 2017 adidas financial statements contain the following selected data (in millions). Compute the following values and provide a brief interpretation of each. (a) Working capital. (b)...
-
Delta Company has 100,000 shares outstanding and plans to pay $1.00 per share in dividends each quarter next year. Delta has a capital budget of $700,000 for next year and plans to maintain its...
-
In your diagnostic work, how often do you diagnose the power terrain of the client system? Once you have the data, what do you use them for in your change work?
-
What is the size of the typical error in predicting the number of home runs, based on the players batting average?
-
Ruiz Engineering Contractors incurred service salaries and wages of $36,000 ($28,000 direct and $8,000 indirect) on an engineering project. The company applies overhead at a rate of 25% of direct...
-
Saint John Mining operates several facilities. At one, a typical batch of an ore, Pryex, run through the processing plant yields three products: PX-10, PX-20, and PX-30. At the split-off point, the...
-
Go to the Webinars worksheet. DeShawn wants to determine the number of webinars the company can hold on Tuesdays and Thursdays to make the highest weekly profit without interfering with...
-
Which of the, two phenols in each set is more acidic? Explain. Phenol or m-chlorophenol
-
Draw the important resonance structures of the radicals formed when each of the following react with R, a general free radical. BHT
-
Moral hazard arises in the insurance market because ________. A. Buyers of insurance have private information that they can use B. Insurance companies can offer a range of premiums to buyers C....
-
Perpetual Inventory Control Record Description: M & B Supreme Date Purchase Received Issued Sales Units Unit Cost June 1 Balance forward 3 $10.00 4 2 6 8 9 $10.50 9 12 32 3 6 2 4 15 6 10 $11.00 18 20...
-
A rectangular footing of size 4m by 5m is founded at 2m below ground level in a uniform deposit of saturated clay. The footing is designed to support a total vertical load of 8000 kN inclusive of the...
-
P6.2 At the start of Tom Stoppard's "Rosencrantz and Guildenstern are dead" 1, Rosencrantz finds a coin. Guildenstern watches as Rosencrantz repeatedly tosses the coin and every time it comes down...
-
For the data: 9 5 10 7 9 10 11 8 12 769 a) Compute the z-score for the raw score of 10 b) Find the raw score that corresponds to z=+1.22
-
(11%) Problem 7: After a bad thunderstorm, a loose power line comes to rest on a parked van. The van is insulated from the ground by its tires, and accumulates an electric charge of Q = 0.0012...
-
Consider a portfolio manager whose portfolio has $5 billion in assets. If this manager could increase the return on the portfolio an additional twotenths of 1 percent, how much would that add to the...
-
Transform the while loop from the previous exercise into an equivalent for loop (make sure it produces the same output).
-
You need to know the melting point for CaC1 2 , for a lab report you are writing. Your lab partner says that the Handbook of Chemistry and Physics lists this as 68 o C. Do you think you should trust...
-
Show a Lewis structure for A1C1 4 . What are the formal charges on the atoms of this anion? What is its shape?
-
Ammonium cyanate is composed of an ammonium caution (NH 4 + ) and a cyanate anion (OCN ). Show a Lewis structure for the cyanate anion. (Both O and N are bonded to C.) Which atom has the negative...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


