Computation of the Prandtl numbers for gases at low density. (a) By using the Eucken formula and
Question:
Computation of the Prandtl numbers for gases at low density.
(a) By using the Eucken formula and experimental heat capacity data, estimate the Prandtl number at 1atm and 300K for each of the gases listed in the table.
(b) For the same gases, compute the Prandtl number directly by substituting the following values of the physical properties into the defining formula Pr = Cpµ/k, and compare the values with the results obtained in (a). All properties are given at low pressure and 300K.
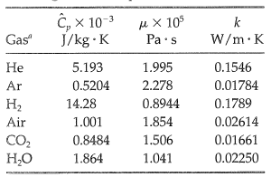
Transcribed Image Text:
t, x 10 μχ 10 J/kg · K Gas Pa·s W/m·K Не 5.193 1.995 0.1546 0.5204 2.278 0.01784 Ar H2 14.28 0.8944 0.1789 1.854 0.02614 Air 1.001 CO, но 1.506 0.01661 0.8484 1.864 1.041 0.02250
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (19 reviews)
Computation of the Prandtl number for gases at low density Us...View the full answer

Answered By

Anthony Ngatia
I have three academic degrees i.e bachelors degree in Education(English & Literature),bachelors degree in business administration(entrepreneurship option),and masters degree in business administration(strategic management) in addition to a diploma in business management.I have spent much of my life in the academia where I have taught at high school,middle level colleges level and at university level.I have been an active academic essays writer since 2011 where I have worked with some of the most reputable essay companies based in Europe and in the US.I have over the years perfected my academic writing skills as a result of tackling numerous different assignments.I do not plagiarize and I maintain competitive quality in all the assignments that I handle.I am driven by strong work ethics and a firm conviction that I should "Do Unto others as I would Like them to do to me".
4.80+
76+ Reviews
152+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Substituting appropriate values for x and y into the formula of Theorem 1.9, show that (a) (b) (c) (-10
-
For liquid metals with Prandtl numbers much less the unity, the hydrodynamic boundary layer is much thinner than the thermal boundary layer. As a result, one may assume that the velocity in the...
-
The density values in Table 12.1 are listed in increasing order. A chemistry student notices that the first four chemical elements that are included are also listed in order of increasing atomic...
-
Data Corporation has four employees and provides group term life insurance coverage for all four employees. Coverage is nondiscriminatory and is as follows: a. How much may Data Corporation deduct...
-
Wegesin and Stem (2004) found greater consistency (less variability) in the memory performance scores for younger women than for older women. The following data represent memory scores obtained for...
-
Two university students, Jim Carstens and Angie Orsini, are planning to open a walk-in pizza restaurant near campus that they will call Big Slice Pizza. They will have no seating and will sell two...
-
10. If f : R ---+ R is continuous and lim f(x) = lim f(x) = 00, x--+oo x--+-oo prove that f has a minimum on R; i.e., there is an Xm E R such that f(xm ) = inf f(x) < 00.
-
The bookstore at State University purchases from a vendor sweatshirts emblazoned with the school name and logo. The vendor sells the sweatshirts to the store for $38 apiece. The cost to the bookstore...
-
Congratulations! You have won a state lottery. The state lottery offers you the following (after-tax) payout options: E: (Click the icon to view the payout options.) (Click the icon to view Present...
-
Mr G is an accountant. Mr. G is 47 years old and is married to Claire who is 45 years old and blind. She has Net Income For Tax Purposes in 2020 of $9,000, all of which is interest on investments she...
-
Prediction of thermal conductivities of gases at tow density, (a) Compute the thermal conductivity of argon at 100C and atmospheric pressure, using the Chapman-Enskog theory and the Lennard-Jones...
-
Estimation of the thermal conductivity of a dense gas, predict the thermal conductivity of methane at 110.4atm and 127F by the following methods: (a) Use Fig. 9.2-1. Obtain the necessary critical...
-
Y = 5x 3
-
At 3 1 st March, 2 0 2 3 , AB Ltd , had an Authorized Capital of K 3 5 , 0 0 0 divided into 1 0 , 0 0 0 7 . 5 % noncumulative per share being due on 3 0 th June, 1 9 6 4 . per share paid, the...
-
A Leadership and Workforce Development Perspective. The literature review should discuss the related literature, organized by topic or themes (not a list of sources). A literature review includes...
-
Critical Success Factors (CSF) are elements that are necessary for an organization or a project to attain its objectives. For example, Chief Executive support is a CSF for corporate sustainability...
-
Ultra Ceramic Products presented the following data for its operations for the month of October, 2020: Dept 1 Work in process, July t. 1(Conversion costs, 60%) 7,000 units Transferred to Dept 2 Work...
-
Choose a global organizational leader who demonstrated how a high level of ethical communication via social media technologies have worked best at building trust with virtual stakeholders. Identify a...
-
A company issues 7000 shares of its $10 par value common stock in exchange for equipment valued at $105,000. The entry to record this transaction includes a credit to (a) Paid-In Capital in Excess of...
-
F.(3e* -2x 3 sin(2x)) is equal to 2 3 Cos 8. IT 3, t (4+@ 2 3, 1+o 1 4 Cos 4 4 1 3. 1 +4cos V7 (1+o 4 1 4 Cos 4 1+0 4-
-
What reagent solution might you use to separate the cations in each of the following mixtures? (a) PbSO 4 (s) and Cu(NO 3 ) 2 (s) (b) Mg(OH) 2 (s) and BaSO 4 (s) (c) PbCO 3 (s) and CaCO 3 (s)
-
The chapter opener suggests that deficits are generally a problem for countries. Based on the models presented in Section 13.3, are there circumstances in which it might be possible that deficits...
-
Use Equation (13.4), repeated below, to demonstrate how an increase in the budget deficit must increase the trade deficit if neither consumption nor investment changes. What must happen if there is...
-
Explain the reasons for the projected increase in the U.S. budget deficit in coming years.
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


