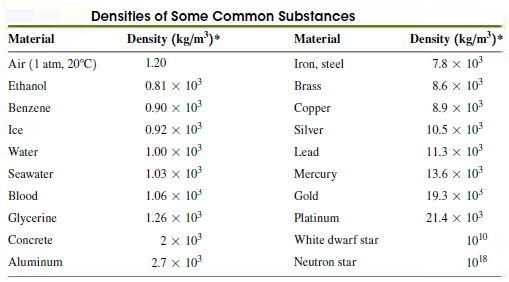
The density values in Table 12.1 are listed in increasing order. A chemistry student notices that the
Question:
(a) See whether there is a simple relationship between density and atomic mass by plotting a graph of density (in g/cm3) versus atomic mass for all eight elements in that table. (See Appendix D for their atomic masses in grams per mole.)
(b) Can you draw a straight line or simple curve through the points to find a €œsimple€ relationship?
(c) Explain why €œMore massive atoms result in more dense solids€ does not tell the whole story.
Table 12.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman
Question Posted:





