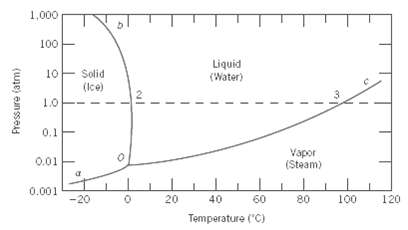
Consider a specimen of ice that is at 210(C and 1 atm pressure. Using Figure, the pressure???temperature
Question:
Consider a specimen of ice that is at 210(C and 1 atm pressure. Using Figure, the pressure???temperature phase diagram for H2O, determine the pressure to which the specimen must be raised or lowered to cause it
(a) To melt, And
(b) To sublime.
Transcribed Image Text:
1,000 100 Liquid (Water) 10F Solid (Ice) 1.0 0.1 Vapor (Steam) 0.01 0.001 -20 20 40 60 80 100 120 Temperature ("C) Fressure (atm)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (20 reviews)
The figure below shows the pressuretemperature phase diagram for H 2 O ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
Consider a specimen of ice that is at -15C and 10 atm pressure. Using Figure 9.2, the pressure-temperature phase diagram for H2O, determine the pressure to which the specimen must be raised or...
-
Determine the distance h to which a hole must be bored into the cylinder so that the center of mass of the assembly is located at x = 64 mm. The material has a density of 8Mg/m3. 120 mm- 40 mm 20 mm
-
Boom member ABC can be raised or lowered by hydraulic cylinderDB. For the position shown, this cylinder pushes on point B with a force of 1650 lb in directionDB as indicated by the dotted line. Find...
-
How does MacKinnon analyze cases of sexual harassment where the woman complies with the sexual advance? She suggests that these cases are therefore not wrongful. She suggests that these cases are too...
-
O'Neill, Incorporated's segmented income statement for the most recent month is given below. For each of the following questions, refer back to the above original data.If Store B sales increase by...
-
The 30-year mortgage rate is now less than 6.0%. A sample of eight financial institutions in Canada showed the mean mortgage rate to be 5.6375%, with a standard deviation of 0.6346%. At the 0.01...
-
Explain the difference between the traditional and integrated marketing. LO.1
-
Ladora Construction Company began operations on January 1, 2019, when it acquired $30,000 cash from the issuance of common stock. During the year, Ladora purchased $6,000 of direct raw materials and...
-
8. Which of the following taxes are deductible for federal income tax purposes as an itemized deduction? a. Ad valorem personal property tax b. FICA tax imposed on employees c. Federal gift tax d....
-
A put option in finance allows you to sell a share of stock at a given price in the future. There are different types of put options. A European put option allows you to sell a share of stock at a...
-
Cite three variables that determine the microstructure of an alloy.
-
At a pressure of 0.01 atm, determine (a) The melting temperature for ice, and (b) The boiling temperature for water.
-
Express the distance to default in terms of the risk-neutral probability of default.
-
In addition to the strongest military in the world, the United States wields enormous soft power. Define soft power. What factors make the United States powerful when it comes to soft power?
-
Tampa by the Bay Cardiology practice is experiencing long wait times for new patient appointments. Next available appointment is 30 days. The administrator has asked the practice manager to construct...
-
In 2013, Idalia Hernndez Ramos, a middle school teacher in Mexico, was a victim of cyber harassment. After discovering that one of her students tweeted that the teacher was a "bitch" and a "whore,"...
-
Your life couldn't be any better. You just accepted a new role as a senior consultant for a project management services firm in San Francisco, and the move is finally happening. You've got a great...
-
What are the two "engines" that drive earth's processes, how do they work (basically) and what are their energy sources? How do the "engines" influence and interact with the Earth Systems? (provide a...
-
Why is an organizations culture perhaps the most evident during crisis situations? LO5
-
You've been asked to take over leadership of a group of paralegals that once had a reputation for being a tight-knit, supportive team, but you quickly figure out that this team is in danger of...
-
Why is energy density so important in the transportation of fuels?
-
Carbon diffuses in iron via an interstitial mechanism-for FCC iron from one octahedral site to an adjacent one. In Section 4.3 (Figure 4.3a), we note that two general sets of point coordinates for...
-
The outer surface of a steel gear is to be hardened by increasing its carbon content; the carbon is to be supplied from an external carbon-rich atmosphere maintained at an elevated temperature. A...
-
An FCC iron-carbon alloy initially containing 0.10 wt% C is carburized at an elevated temperature and in an atmosphere in which the surface carbon concentration is maintained at 1.10 wt%. If after 48...
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


