Question: Consider the solidification of a binary alloy with the phase diagram of Figure. Show that, regardless of the initial composition, the melt will always become
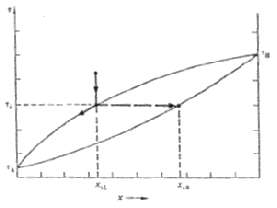
Consider the solidification of a binary alloy with the phase diagram of Figure. Show that, regardless of the initial composition, the melt will always become fully depleted in component B by the time the last remnant of the melt solidifies. That is, the solidification will not be complete until the temperature has dropped toTA.
i 1
Step by Step Solution
3.35 Rating (173 Votes )
There are 3 Steps involved in it
Qualitatively the claimed result is all but obvious when the last infinitesimal remnant of liquid solidifies the solid forming from this remnant must ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
42-P-S-S-T-T (107).docx
120 KBs Word File


