Question: Derive Equation 6.5-4 for the boiling-point elevation of a dilute solution of a nonvolatile solute with mole fraction x in a solvent that has a
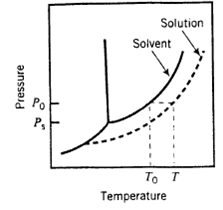
Derive Equation 6.5-4 for the boiling-point elevation of a dilute solution of a nonvolatile solute with mole fraction x in a solvent that has a pure-component vapor pressure ps (T). To do so, suppose that when the pressure is P0, the pure solvent boils at temperature Tb0 (so that P0 = ps (Tb0)] and the solvent in the solution boils at Tbs > Tb0. Further suppose that at temperature T the effective vapor pressure of the solvent is Ps = (ps)(Tb0) < P0. (See diagram.) The procedure is as follows.
(a) Write the Clausius—Clapeyron equation (Equation 6.1-3) for Ps (the effective solvent vapor pressure at Tb0) and then for P0 (the effective solvent vapor pressure at Tbs), assuming that at the low solute concentrations in question the heat of vaporization is the same at both temperatures. Subtract the two equations. Simplify the equation algebraically assuming that T and T are close enough together to say that Tb0 Tbs ≈ T2b0.
(b) Substitute the Raoult’s law expression (Equation 6.5-2) for Ps = (ps) e (Tb0). Observe that if x << 1 (which it is for highly dilute solutions), then in (1 – x) ≈ – x. The desired result follows.
Solution Solvent Po P, To Temperature Pressure
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
RT 50 Assume a 1npTo B 1n... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (339).docx
120 KBs Word File


