Diethylstilbestrol (DES) has estrogenic activity even though it is structurally unrelated to steroids. Once used as an
Question:
Diethylstilbestrol (DES) has estrogenic activity even though it is structurally unrelated to steroids. Once used as an additive in animal feed, DES has been implicated as a causative agent in several types of cancer. Show how DES can be drawn so that it is sterically similar toestradiol.
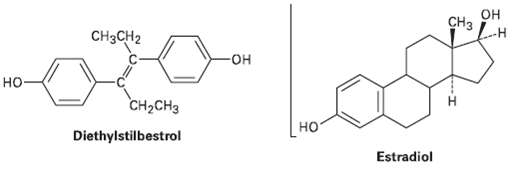
Transcribed Image Text:
Cнз он CH3CH2 но CH-CHз но Diethylstilbestrol Estradiol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (10 reviews)
HO H Estradiol HT CH3OH H CH3 Logo HC D...View the full answer

Answered By

Mario Alvarez
I teach Statistics and Probability for students of my university ( Univerisity Centroamerican Jose Simeon Canas) in my free time and when students ask for me, I prepare and teach students that are in courses of Statistics and Probability. Also I teach students of the University Francisco Gavidia and Universidad of El Salvador that need help in some topics about Statistics, Probability, Math, Calculus. I love teaching Statistics and Probability! Why me?
** I have experience in Statistics and Probability topics for middle school, high school and university.
** I always want to share my knowledge with my students and have a great relationship with them.
** I have experience working with students online.
** I am very patient with my students and highly committed with them
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Is VCW Inc. a social enterprise even though it is a for-profit business? On what do you base your answer? Is Cheryl Womack a social enterpriser?
-
Show how dimedone can be synthesized from malonic ester and 4-methyl-3-penten-2-one (mesityl oxide) under basic conditions. OH Dimedone
-
Show how 1-butanol can be converted into the following compounds: a. CH3CH2CH2CH2Br b. c. CH3CH2CH2CH2OCH3 d. CH3CH2CH2CH2NHCH2CH3 e. CH3CH2CH2CH2SH f. CH3CH2CH2CH2C==N CH CH2CHCH2OCCH2CH,
-
Simplity each of the follewing ratios. f r 15 15kg:350 g 0.45:085 ( 580 ml: L121:104 m/ 40 033:063: 18
-
1. Why would Alibaba choose to list in the U.S. and not in the Hong Kong Exchange? 2. The Hong Kong Exchange rejected Alibaba's application to list on it because of its corporate governance...
-
Exhibits 1.26??1.28 of Integrative Case 1.1 (Chapter 1) present the financial statements for Walmart for 2012 to 2015. In addition, the website for this text contains Walmart??s December 31, 2015,...
-
Consider human safeguards in particular: a. Explain why the data loss resulted from a lack of human safeguards. b. Even though MRV is small, describe employee termination procedures as they relate to...
-
The Far North Centre (the Centre) is an anti-poverty organization funded by contributions from governments and the general public. For a number of years it has been run by a small group of permanent...
-
Chapter 5 Homework i Seved Help Save & Exit Submit Check my work 07 2 points Thermal Rising, Inc., makes paragliders for sale through specialty sporting goods stores. The company has a standard...
-
Grady Zebrowski, age 25, just graduated from college, accepted his first job with a $50,000 salary, and is already looking forward to retirement in 40 years. He assumes a 3 percent inflation rate and...
-
Diterpenoids are derived biosynthetically from geranyl-geranyl diphosphate (GGPP), which is usd1 biosynthesized by reaction of farnesyl diphosphate with isopentenyl diphosphate. Show the structure of...
-
Propose a synthesis of diethylstilbestrol (Problem 27.44) from phenol and any other organic compound required.
-
EveryWhere Development Co. showed the following selected PPE balances on January 31, 2014: Van............................................................................................ $64,400...
-
In the global discourse on healthcare, the United States and England stand out as two contrasting models, each providing a distinct approach to addressing the challenges of cost , access, and...
-
2.A. Using the quotes below, answer the following questions. Exchange rate Bid Ask In New York, USD/EUR 1.2267 1.2875 In London, USD/GBP 1.6555 1.7334 2.A1. Calculate the EUR/GBP cross exchange...
-
Question 43 Part B Q1ii 20 points Save A a) A property is currently leased for $100,000 p.a. with fully recoverable outgoings. The lease has 3 years to run on the current (fixed) rent. The market...
-
Define HIPPA? What is the purpose of HIPPA? What are the 4 main rules of HIPPA?
-
Accounting for Inventories" Please respond to the following: As a Financial Accountant,determine the best type of income statement a retailer should use.Defend your suggestion. Analyze inventory...
-
Name and describe the different types of hotels and lodging. LO.1
-
Copy and complete the statement. 3800 m ? km =
-
The evaporation of a 120-nm film of n-pentane from a single crystal of aluminum oxide is zero order with a rate constant of 1.92 * 10 13 molecules/cm 2 s at 120 K. a. If the initial surface coverage...
-
Starting with -d-glucose and using acid (H1) as a catalyst, write out all of the steps in the mechanism for the mutarotation process.
-
Lactose exists in a and b forms, with specific rotations of 192.6 and 134, respectively. a. Draw their structures.
-
Oxidation of either d-erythrose or d-threose with nitric acid gives tartaric acid. In one case, the tartaric acid is optically active; in the other, it is optically inactive. How can these facts be...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


