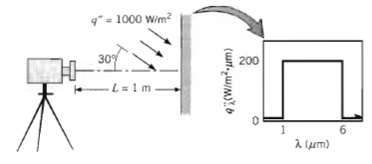
A diffuse, opaque surface at 700 K has spectral emissivities of ?? = 0 for 0 1000
Question:
A diffuse, opaque surface at 700 K has spectral emissivities of ?? = 0 for 0
Transcribed Image Text:
1000 Wim? 30% L= 1 m 6. (urti u/m)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
KNOWN Temperature and spectral emissivity of a receiving surface Direction and spectral dis...View the full answer

Answered By

Isaiah Mutinda
As a graduate with Bs in Maths and Computer Science and having worked as a freelance full stack software developer for 3 years running I believe I have what it takes to conformable tutor and mentor a student to a professional developer also.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Heat and Mass Transfer
ISBN: 978-0471457282
6th Edition
Authors: Incropera, Dewitt, Bergman, Lavine
Question Posted:
Students also viewed these Mechanical Engineering questions
-
At 700 K the equilibrium constant for the reaction Is Kp= 0.76. A flask is charged with 2.00 atm of CCl4, which then reaches equilibrium at 700 K. (a) What fraction of the CCl4 is converted into C...
-
The spectral emissivity of an opaque surface at 1500 K is approximated as Determine the total emissivity and the emissive flux of thesurface. for <2 m | -0 0.85 ) for 2 6 for for >6 =0
-
For materials A and B, whose spectral hemispherical emissivities vary with wavelength as shown below, how does the total, hemispherical emissivity vary with temperature? Explain briefly. 2 B
-
Chemistry A one-electron atom is an atom with Z protons in the nucleus and one electron. For example, Z = 2 for helium and Z = 3 for lithium. Use our class discussion of the allowed radii and...
-
Revise the following to make the tone conversational yet professional. 1. Pertaining to your request, the above-referenced items (printer toner and supplies) are being sent to your Oakdale office, as...
-
Quinton Johnston is evaluating TMI Manufacturing Company, Ltd., which is headquartered in Taiwan. In 2008, when Johnston is performing his analysis, the company is unprofitable. Furthermore, TMI pays...
-
5. Let r > 0, a, bE R, f : Br (a, b) ---t R be differentiable, and (x, y) E Br (a, b). (a) Compute the derivative of g(t) = f(tx + (1 - t)a, y) + f (a, ty + (1 - t)b). (b) Prove that there are...
-
Western Farms is a producer of items made from local farm products that are distributed to supermarkets. Over the years, price competition has become increasingly important, so Doug Gilbert, the...
-
PETRON Company purchased 6 0 % of the outstanding shares of SHELL Company by paying P 3 0 0 , 0 0 0 on January 2 , 2 0 2 2 . SHELL had Share Capital and Retained Earnings on this date amounting to P...
-
Nightwish Corp. shows the following information on its 2021 income statement: Sales = $336,000; Costs = $194,700; Other expenses = $9,800; Depreciation expense = $20,600; Interest expense = $14,200;...
-
An opaque surface, 2 m by 2 m, is maintained at 400 K and is simultaneously exposed to solar irradiation with G = 1200 W/m2 . The surface is diffuse and its spectral absorptivity is = 0, 0.8, 0, and...
-
A small disk 5 mm in diameter is positioned at the center of an isothermal hemispherical enclosure. The disk is diffuse and gray with an emissivity of 0.7 and is maintained at 900 K. The...
-
An ancient skull has a carbon-14 decay rate of 0.85 disintegration per minute per gram of carbon (0.85 dis/min g C). How old is the skull? (Assume that living organisms have a carbon-14 decay rate...
-
Given forecast errors of 4, 8, and -3, what is the MAD? What is the MSE?
-
Padgett Rentals can purchase a van that costs \($48,000\) ; it has an expected useful life of three years and no salvage value. Padgett uses straight-line depreciation. Expected revenue is...
-
Rainwater Corp. expects to sell 600 umbrellas in May and 400 in June. Each umbrella sells for \($15\). Rainwaters beginning and ending finished goods inventories for May are 75 and 50 units,...
-
Don Moon is the owner of ABC Cleaning. At the beginning of the year, Moon had \(\$ 2,400\) in inventory. During the year, Moon purchased inventory that cost \(\$ 13,000\). At the end of the year,...
-
Agua Ole is a distributor of bottled water. For each of items a through c, compute the amount of cash receipts or payments Agua Ol will budget for September. The solution to one item may depend on...
-
Many cost-driver rates of the target-costing analysis in Exhibit 5-2 are taken directly from the original ABC analysis of existing products. Several cost-driver rates are notably different, however,...
-
For the data in Exercise 17-19, use the FIFO method to summarize total costs to account for, and assign these costs to units completed and transferred out, and to units in ending work in process....
-
Use bond energies from Table 10.3 to determine H rxn for the reaction between ethanol and hydrogen chloride. CHCHOH(g) + HCI(g) CHCHCl(g) + HO(g) b) 1549 kJ c) -12 kJ d) 12 kJ a) -1549 kJ
-
A flat-belt drive is to consist of two 4-ft-diameter cast-iron pulleys spaced 16 ft apart. Select a belt type to transmit 60 hp at a pulley speed of 380 rev/min. Use a service factor of 1.1 and a...
-
In solving problems and examining examples, you probably have noticed some recurring forms
-
Return to Ex. 171 and complete the following. (a) Find the torque capacity that would put the drive as built at the point of slip, as well as the initial tension Fi . (b) Find the belt width b that...
-
Regarding research and experimental expenditures, which of the following are not qualified expenditures? 3 a. costs of ordinary testing of materials b. costs to develop a plant process c. costs of...
-
Port Ormond Carpet Company manufactures carpets. Fiber is placed in process in the Spinning Department, where it is spun into yarn. The output of the Spinning Department is transferred to the Tufting...
-
Oct. 31: Paid salaries, $45,000 ( 75% selling, 25% administrtive). Data table Data table them to retail stores. The company has three inventory items: and floor lamps. RLC uses a perpetual inventory...

Study smarter with the SolutionInn App


