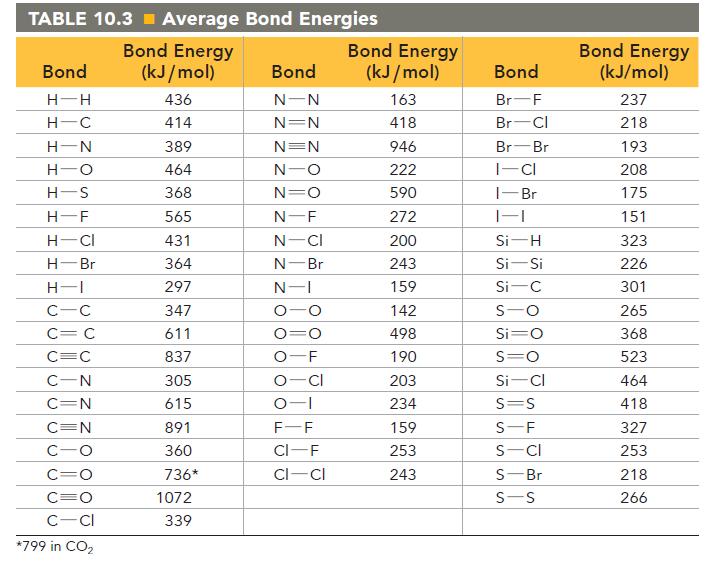
Use bond energies from Table 10.3 to determine H rxn for the reaction between ethanol and hydrogen
Question:
Use bond energies from Table 10.3 to determine ΔHrxn for the reaction between ethanol and hydrogen chloride.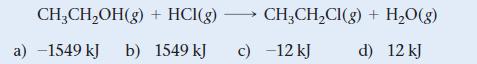
Transcribed Image Text:
CH₂CH₂OH(g) + HCI(g) →→→→→ CH₂CH₂Cl(g) + H₂O(g) b) 1549 kJ c) -12 kJ d) 12 kJ a) -1549 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
c...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Resonance energy is the difference in energy between a real moleculea resonance hybridand its most important contributing structure. To determine the resonance energy for benzene, we can determine an...
-
a. Write a balanced equation to show the reaction between ethanol and hydrogen bromide. b. What are the reagents and conditions used for this reaction? c. What do we call this type of reaction?
-
Hydrazine (N 2 H 4 ) is used as a fuel in liquid-fueled rockets. When hydrazine reacts with oxygen gas, nitrogen gas and water vapor are produced. Write a balanced equation and use bond energies from...
-
A 14-foot piece of string is cut into two pieces so that the longer piece is 2 feet longer than twice the shorter piece. Find the lengths of both pieces. What is the lenath of the shorter oiece?1...
-
Whats going on in that lab? asked Derek Warren, chief administrator for Cottonwood Hospital, as he studied the prior months reports. Every month the lab teeters between a profit and a loss. Are we...
-
A partial list of trend and common-size percentage for ABC Ltd. for years 1 and 2 is given below: (a) Determine the missing trend and common-size percentages. (b) Compute the net income for year 2....
-
Discuss how the devils advocacy technique and the dialectic model encourage functional conflict.
-
You are a CA who has just been hired by Saskatoon Fields Forever Inc. (SFF), formerly MUGS Partnership (MUGS) as the controller. It is August 1, 2014, your rst day at SFF. You are meeting with Meghan...
-
View previous attempt Chech 6 The Caldwell Herald newspaper reported the following story: Frank Ormsby of Caldwell is the state's newest millionaire. By choosing the six winning numbers on last...
-
Brava Landscaping, Inc., completed the following transactions during its first month of operations for January 2016: a. Gabrielle Brava invested $8,500 cash and a truck valued at $16,000 to start...
-
In a covalent Lewis structure, what is the difference between lone pair and bonding pair electrons?
-
How does lattice energy relate to ionic radii? To ion charge?
-
Repeat Exercise 6.7, but select each sample with replacement; that is, replace each slip of paper and reshuffle before the next one is drawn. Data From Exercise 6.7 6.7 Take 30 slips of paper and...
-
Compare the alternatives that Bergerac is considering for its decision. Include: Comparison of make versus buy option in the type of operation that Bergerac is looking to integrate. You do not need...
-
Let A, B, C and D be non-zero digits, such that CD is a two-digit positive integer. BCD is a three-digit positive integer generated by the digits B, C and D. ABCD is a four-digit positive integer...
-
1.) An aluminum tube is clamped with rigid plates using four bolts as shown. The nut on each bolt is tightened one turn from 'snug'. The thickness of the plate may be considered insignificant in this...
-
4.21 Case Study Competency IV.1RM Determine diagnosis and procedure codes and groupings according to official guidelines. Competency IV.1 Validate assignment of diagnostic and procedural codes and...
-
W.E.B Dubois taught the book called "The State" to his students at Atlanta University. Who wrote this book
-
An electrochemical cell is constructed such that on one side a pure nickel electrode is in contact with a solution containing Ni2+ ions at a concentration of 3 10-3 M. The other cell half consists...
-
Write a function that reads a Float24_t value: Float24_t float24_read(void) A legitimate float24 value string is of the form: "mantissabexponent" where the mantissa (m) and the exponent (e) may have...
-
A shear force of V = 18 kN is applied to the box girder. Determine the shear flow at point C. 10 mm 30 mm 10 mm 100 mm B. 150 mm 100 mm 10 mm 30 mm 10 mm 150 mm 10 mm 125 mm 10 mm
-
The aluminum strut is 10 mm thick and has the cross section shown. If it is subjected to a shear of V = 150 N, determine the shear flow at points A and B. 10 mm 40 mm 10 mm t+-40 mm- 30 mm 30 mm 10...
-
The aluminum strut is 10 mm thick and has the cross section shown. If it is subjected to a shear of V = 150 N, determine the maximum shear flow in the strut. 10 mm 40 mm 10 mm tr-40 mm- 30 mm 30 mm...
-
true- false statement (1) Present value is additive. G) Publie firms smooth dividends to satisfy shareholders' consumption preferences
-
Restate the following one-, three-, and six-month outright forward European term bid-ask quotes in forward points. Spot 1.3515 1.3532 One-Month 1.3528 1.3550 Three-Month 1.3544 1.3571...
-
The Storm Soccer Team sells season tickets and collects the cash in January at the beginning of the season. The team collected $57,750 for season tickets. The soccer season starts in February and the...

Study smarter with the SolutionInn App


