Hydrazine (N 2 H 4 ) is used as a fuel in liquid-fueled rockets. When hydrazine reacts
Question:
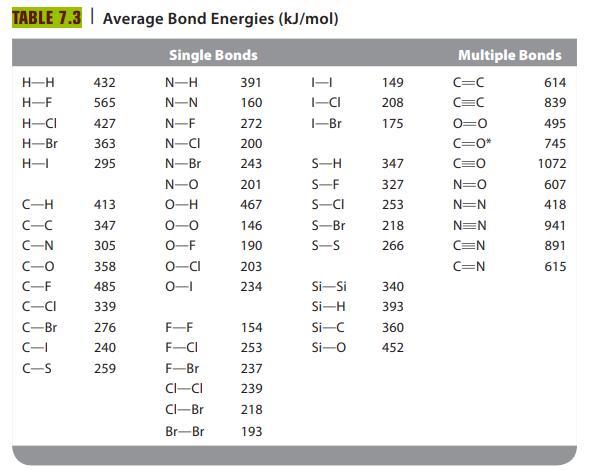
Hydrazine (N2H4) is used as a fuel in liquid-fueled rockets. When hydrazine reacts with oxygen gas, nitrogen gas and water vapor are produced. Write a balanced equation and use bond energies from Table 7.3 to estimate ΔH for this reaction.
Transcribed Image Text:
TABLE 7.3 Average Bond Energies (kJ/mol) Single Bonds N-H N-N H-H H-F H-CI H-Br H-1 C-H C-C C-N C-O C-F -CI C-Br C-I C-S 432 565 427 363 295 413 347 305 358 485 339 276 240 259 N-F N-CI N-Br N-O O-H 0-0 O-F O-CI 0-1 F-F F-CI F-Br CI-CI Cl-Br Br-Br 391 160 272 200 243 201 467 146 190 203 234 154 253 237 239 218 193 H I-CI 1-Br S-H S-F S-CI S-Bri S-S Si-Si Si-H Si-C Si-O 149 208 175 347 327 253 218 266 340 393 360 452 Multiple Bonds C=C C=C 0=0 C=O* C=0 N=O N=N N=N C=N C=N 614 839 495 745 1072 607 418 941 891 615
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The balanced chemical equation for the reaction between hydrazine N2H4 and oxygen ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Write an equation for the reaction of hydrazine with fluorine gas to produce nitrogen gas and hydrogen fluoride gas. Estimate ÎH for this reaction, using bond energies from Table 13.6. Table...
-
A promising new material with great potential as a fuel in solid rocket motors is ammonium dinitramide [NH4N(NO2)2]. a. Draw Lewis structures (including resonance forms) for the dinitramide ion...
-
The steering rockets in space vehicles use N 2 O 4 and a derivative of hydrazine, 1,1-dimethylhydrazine (Study Question 5.86). This mixture is called a hypergolic fuel because it ignites when the...
-
Problem 3.6.1 Given the random variable Y in Problem 3.4.1, let U = g(Y) = Y2. (a) Find Pu(u). (b) Find Fu(u). (c) Find E[U].
-
Powderhorn Corporation reported sales of $257,000, net income of $45,300, cash of $9,300, and net cash provided by operating activities of $21,200. Accounts receivable have increased at three times...
-
What must a plaintiff prove to recover for defamation?
-
P Ltd is in receipt of periodic payments in respect of patent royalties and pays debenture interest twice in each accounting period. Income tax is deducted at source from both the royalties and the...
-
A manufacturer of printed circuit boards uses exponential smoothing with trend to forecast monthly demand of its product. At the end of December, the company wishes to forecast sales for January. The...
-
James has a desire to prepay his rent ahead of the period in question. On February 1st of 2021 he writes a check for $3,000 to cover the cost of rent for the months of March through May 2021. What...
-
Walgreen Company is a well-known drugstore chain. A condensed balance sheet for August 31, 2011, follows ($ in millions): Use a format similar to Exhibit to analyze the following transactions for the...
-
Although nitrogen trifluoride (NF 3 ) is a thermally stable compound, nitrogen triiodide (NI 3 ) is known to be a highly explosive material. NI 3 can be synthesized according to the equation a. What...
-
Give the Lewis structure, molecular structure, and hybridization of the oxygen atom for OF 2 . Would you expect OF 2 to be a strong oxidizing agent like O 2 F 2 discussed in Exercise 61? Exercise 61....
-
If the maximum allowable tension in cables AB and AC is 500 lb, determine the maximum height z to which the 200-lb crate can be lifted. What horizontal force F must be applied? Take y = 8ft. 4 ft
-
Winston Electronics reported the following information at its annual meetings. The company had cash and marketable securities worth $1,235,740, accounts payables worth $4,160,391, inventory of...
-
Hooray Company has been manufacturing 12,000 units of Part A which is used to manufacture one of its products. At this level of production, the cost per unit is as follows: Direct materials P 4.80...
-
At the beginning of the period, the Grinding Department budgeted direct labor of $171,200 and property tax of $57,000 for 10,700 hours of production. The department actually completed 12,800 hours of...
-
The following information is available for Shamrock Corporation for the year ended December 31, 2025. Beginning cash balance $ 58,500 Accounts payable decrease 4,810 Depreciation expense 210,600...
-
In today's stock market, compounding is the key to making money in the future for one's investments. However, with decentralized currency growing rapidly (Crypto), how can one rely on TVM for FV...
-
How is mobility restricted using WLANs? What additional elements are needed for roaming between networks, how and where can WLANs support roaming? In your answer, think of the capabilities of layer 2...
-
In Problems 718, write the augmented matrix of the given system of equations. f0.01x0.03y = 0.06 [0.13x + 0.10y = 0.20
-
VSEPR (valence state electron pair repulsion) theory was formulated to anticipate the local geometry about an atom in a molecule (see discussion in Section 25.1). All that is required is the number...
-
Chemists recognize that the cyclohexyl radical is likely to be more stable than the cyclopentylmethyl radical, because they know that six-membered rings are more stable than five-membered rings and,...
-
The presence of the carbonyl group in a molecule is easily confirmed by an intense line in the infrared spectrum around 1700 cm 1 that corresponds to a CO stretching vibration. Locate this line in...
-
XYZ Corp. applies manufacturing overhead costs to products at a budgeted indirect-cost rate of $65 per direct manufacturing labor-hour. A retail outlet has requested a bid on a special order of a...
-
What did you observe to be the major causes for the volatile week in stock trading this past week?
-
Analyze why there was underpricing or overpricing on listing price for Change Healthcare (CHNG)

Study smarter with the SolutionInn App


