Chemistry An Atoms First Approach 2nd Edition Steven S. Zumdahl, Susan A. Zumdahl - Solutions
Unlock the comprehensive solutions to "Chemistry: An Atoms First Approach, 2nd Edition" by Steven S. Zumdahl and Susan A. Zumdahl with our online answers key. Access detailed chapter solutions, solved problems, and a test bank with questions and answers. Our solution manual provides step-by-step answers, making it an invaluable instructor manual and textbook companion. Discover the ease of finding solutions in a downloadable PDF format, all available for free download. Enhance your understanding with expertly crafted online solutions and make chemistry learning seamless and effective.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()




![a. pOH = 11.21 b. pH = 9.42 c. [OH-] style=](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/8/7/4/3496552062d103cb1699874348327.jpg)
![a. pH = 3.04 b. [H]> 1.0 10-7 M c. pOH = 4.51 d. [OH-] 3.21 10- M](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/8/7/4/286655205eee62891699874287231.jpg)
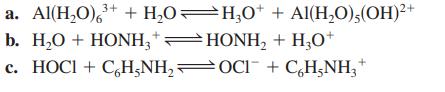
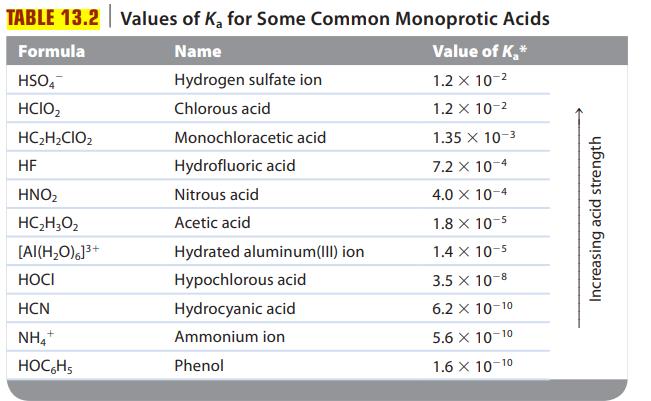
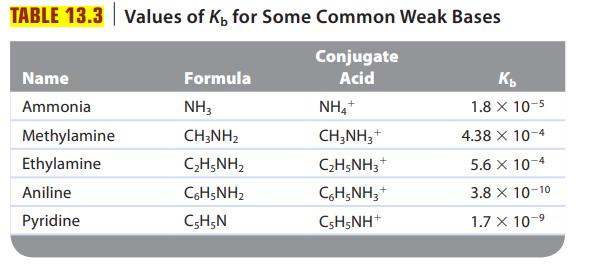
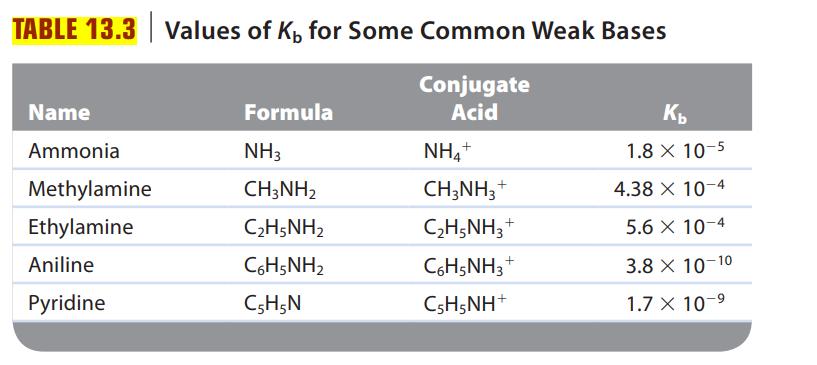
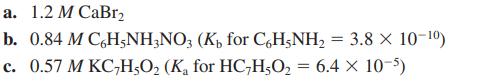
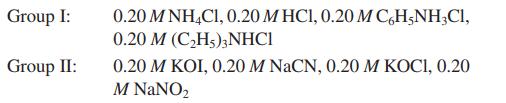

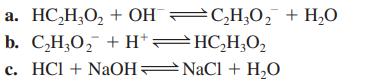
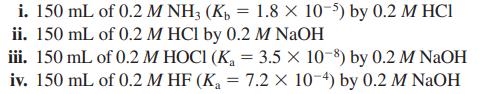
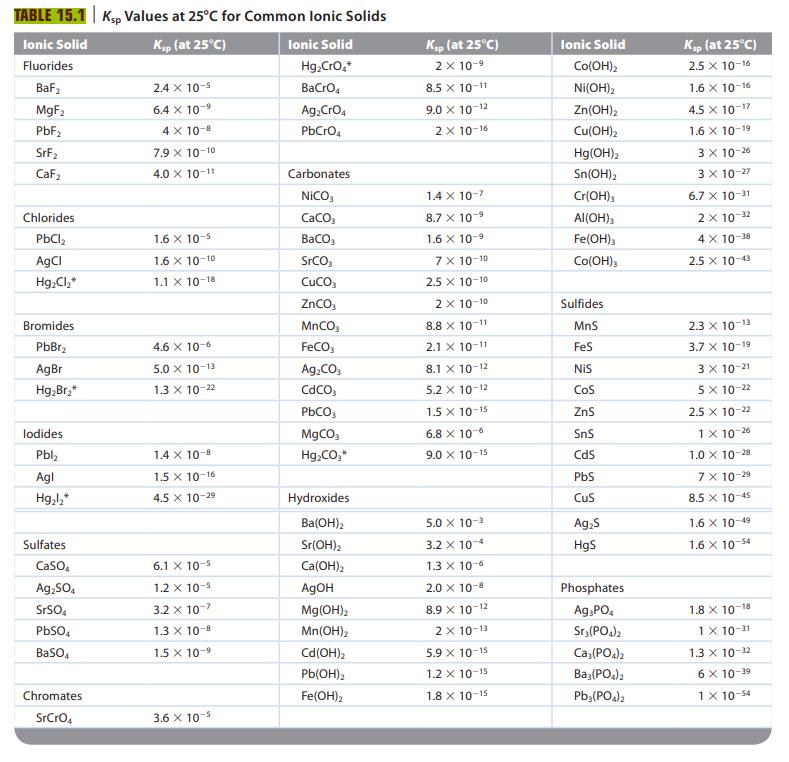
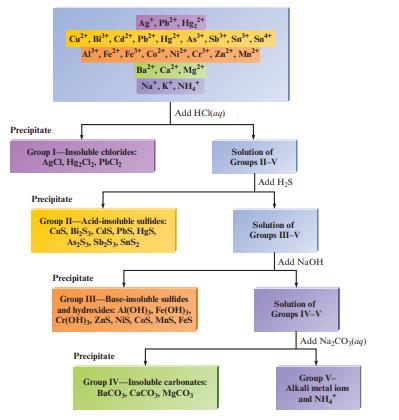

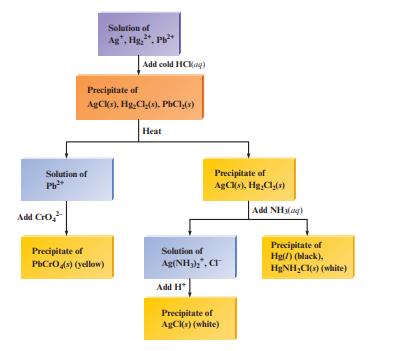
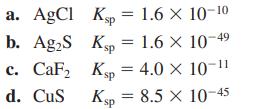
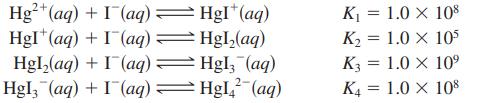
![a. Calculate the equilibrium concentration of [HgI4]. b. Calculate the equilibrium concentration of [I]. c.](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/9/6/4/82265536796b3c181699964823549.jpg)
![[,] 4.2 10-5 2.8 10-5 2.0 10-5 1.4 10-5 1.0 10-5 Time Period (s) 0 50 50 100 100 150 150200 200250](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/7/0/6/782654f779e2c5b81699706783039.jpg)
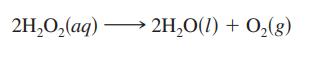
![Time (s) 0 2.16 X 104 4.32 X 104 [HO] (mol/L) 1.000 0.500 0.250](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/7/0/7/417654f7a199abbf1699707418487.jpg)