Fig. 15.3 outlines the classic scheme for separating a mixture of insoluble chloride salts from one another.
Question:
Fig. 15.3 outlines the classic scheme for separating a mixture of insoluble chloride salts from one another. Explain the chemistry involved in the various steps of the figure.
Fig. 15.3
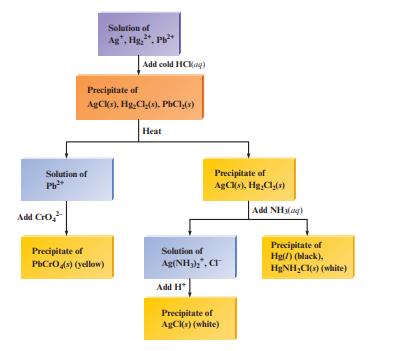
Transcribed Image Text:
Solution of Pb²+ Add CrO₂²- Solution of Ag, Hg., Pb²+ Precipitate of AgCl(s), Hg,Cl(s), PbCl(s) Precipitate of PbCrO4(s) (yellow) Add cold HCKag) Heat Precipitate of AgCl(s), Hg₂Cl(s) Solution of Ag(NH3)2, CT Add H Precipitate of AgCl(s) (white) Add NH3(g) Precipitate of Hg(/) (black). H₂NH₂Cl(s) (white)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The figure you sent me appears to show a separation scheme for a mixture of three ca...View the full answer

Answered By

Rukhsar Ansari
I am professional Chartered accountant and hold Master degree in commerce. Number crunching is my favorite thing. I have teaching experience of various subjects both online and offline. I am online tutor on various online platform.
5.00+
4+ Reviews
17+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Fig. 15.2 summarizes the classic method for separating a mixture of common cations by selective precipitation. Explain the chemistry involved with each of the four steps in the diagram. Fig. 15.2...
-
Outline a procedure for separating a mixture of benzoic acid, 4-methylphenol, aniline,and benzene using acids, bases, and organic solvents.
-
Devise a procedure for separating a mixture of the four stereoisomers of isoleucine into its four components: (+)-isoleucine, (-)-isoleucine, (+)-alloisoleucine, and (-2)-alloisoleucine (Problem 31)....
-
Each of the systems in Problems 11 through 18 has a single critical point (x 0 , y 0 ). Apply Theorem 2 to classify this critical point as to type and stability. Verify your conclusion by using a...
-
If the claimants had refuted the governments assertion that the cash was illicit, would the result in this case have been different? Why or why not?
-
How much heat is needed to vaporize 20.0 mL of liquid methanol, CH3OH, at 25.0C? The density of the liquid is 0.787 g/mL. Use standard heats of formation, which are given in Appendix C.
-
Reflecting back to the chapter opening A Supervision Challenge case, consider how the leadership theories and principles from this chapter might help supervisors lead employees through a difficult...
-
Draw an ISD for the DFD in exercise C in Chapter 5.
-
Limitations of the income statement include all of the following except income numbers are affected by the accounting methods employed w items that cannot be measured reliably are not reported only...
-
The bar codes above represent locations in a warehouse. How many units would be left in each location? If you picked 250 units from location 00000113774360000 If you picked 250 units from location 00...
-
Calculate the molar solubility of Cd(OH) 2 , K sp = 5.9 10 -11 .
-
Under what circumstances can you compare the relative solubilities of two salts directly by comparing the values of their solubility products? When can relative solubilities not be compared based on...
-
Consider the fair price of a European plain vanilla binary contract in the Black Scholes-framework. What is the delta of a binary call and put option?
-
Question 1 Consider the function g (x) = x-6x+1. The discriminant is [Select] [Select] and therefore the graph has x-intercepts.
-
Han Wu Manufacturing uses a job order cost system and applies overhead to production on the basisof direct labour hours. On January 1, 2023, Job no, 50 was the only job in process. The costs incurred...
-
Mr. A Background Mr. A is a 42-year-old divorced man assessed at intake to a crisis intervention unit. He was brought to the facility by the local law enforcement agency after his father complained...
-
QUESTION 1 The bank reconciliation statement of Honshu Ltd for February 2020 is set out below: 1) Bank reconciliation statement on 29 February 2020: < DR Balance as per bank statement Add:...
-
what do you think is the most important thing that adults can do to facilitate physical and motor development? Why?
-
Evaluate the differences in approaches to labor- management relations between the George W. Bush and Obama administrations?
-
When the Department of Homeland Security created a color-coded system to prepare government officials and the public against terrorist attacks, what did it do right and what did it do wrong?
-
Draw a mechanism for the following transformation: NaOH, heat
-
The reaction in the previous problem is an equilibrium process. Draw a mechanism of the reverse process. That is, draw a mechanism showing conversion of the conjugated, cyclic enone into the acyclic...
-
When 2,6-heptanedione is heated in the presence of aqueous sodium hydroxide, a condensation product with a six-membered ring is obtained. Draw the product and show a mechanism for its formation.
-
1. UNIS Let's start with an easy one! Which of the following wou with an easy one! Which of the following would most likely be a fixed cost for a Subway restaurant? a. Lettuce b. Fritos chips c....
-
help please! Requirement 2. Journalize any required entries from the bank reconciliation. (Record debits first, then credits. Se Begin with the EFT collection, Date Credit Debit Accounts and...
-
Pear, an individual, plans to start a small business, which will operate as a corporation. In year 0, she expects the corporation to generate an ordinary loss of $1,550,000. Subsequently, she expects...

Study smarter with the SolutionInn App


