Fig. 15.2 summarizes the classic method for separating a mixture of common cations by selective precipitation. Explain
Question:
Fig. 15.2 summarizes the classic method for separating a mixture of common cations by selective precipitation. Explain the chemistry involved with each of the four steps in the diagram.
Fig. 15.2
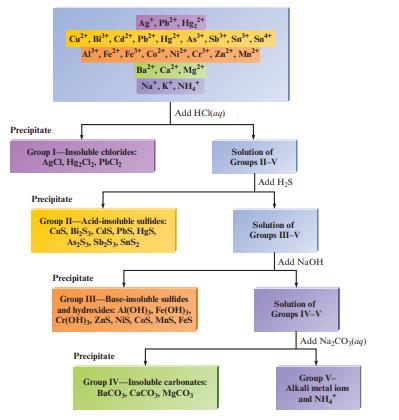

Transcribed Image Text:
Precipitate Ag", Pb²*, Hg: Cu²*, Bi³¹, Cd²*, Pb²+, Hg, As". Sb³*, Sn. AP, Fe, Fe, Co, Ni, Cr³, Zn, Mn Ba, Ca, Mg Na, K, NH, Group 1-Insoluble chlorides: AgCl, HgCl₂, PbCl₂ Precipitate Group II-Acid-insoluble sulfides: CuS, Bi S3, CdS, PbS, H₂S, As2S₁, Sb₂S3, SS₂ Precipitate Add HCl(aq) Precipitate Group III-Base-insoluble sulfides and hydroxides: Al(OH), Fe(OH)3, Cr(OH), ZS, NIS, COS, MuS, Fes Group IV-Insoluble carbonates: BaCO,, CaCO,, MgCO, Solution of Groups II-V Add H₂S Solution of Groups III-V Add NaOH Solution of Groups IV-V Add NaCO3(aq) Group V- Alkali metal ions and NH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
In step one the cations react with HCl to form corresponding salts Salts of AgCl 2 HgC...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Fig. 15.3 outlines the classic scheme for separating a mixture of insoluble chloride salts from one another. Explain the chemistry involved in the various steps of the figure. Fig. 15.3 Solution of...
-
Devise a procedure for separating a mixture of the four stereoisomers of isoleucine into its four components: (+)-isoleucine, (-)-isoleucine, (+)-alloisoleucine, and (-2)-alloisoleucine (Problem 31)....
-
Chromatography (Figure 1.14) is a simple but reliable method for separating a mixture into its constituent substances. You have a mixture of two vegetable dyes, one red and one blue, that you are...
-
In Exercises 2748, find the open intervals where the functions are concave upward or concave downward. Find any inflection points. f(x) = 2e x2
-
What If the Facts Were Different? Suppose that Gladys had told Clara that she was giving the rings to Clara but wished to keep them in her possession for a few more days. Would this have affected the...
-
Identify the phase transition occurring in each of the following. a. The water level in an aquarium tank falls continuously (the tank has no leak). b. A mixture of scrambled eggs placed in a cold...
-
In which of the following situations would you recommend that the supervisor use an authoritarian style of leadership? In which situations would you recommend a democratic style? Explain your...
-
You are the vice-president of finance of Sandy Alomar Corporation, a retail company. The company prepared two different schedules of gross margin for the first quarter ended March 31, 2008. These...
-
You are given the following term structure of interest rates for the dollar and the euro: Term (days) $ Rate (r$) Rate (r)_ DBP$ DBPE 180 2.50% 6.80% 360 2.60% 6.60% 540 2.80% 6.40% 720 3.00% 6.20% I...
-
Mike Curtains, a Registered Tax Agent, attends to the tax affairs of Frodo West. In preparing Frodo's 2021/22 income tax return, the following occurred: Frodo attended a meeting with Mike in August...
-
Under what circumstances can you compare the relative solubilities of two salts directly by comparing the values of their solubility products? When can relative solubilities not be compared based on...
-
To what reaction does the solubility product constant, Ksp, refer? Table 15.1 lists K sp values for several ionic solids. For any of these ionic compounds, you should be able to calculate the...
-
Use the Principle of Mathematical Induction to show that the given statement is true for all natural numbers. 13 + 24 + 35 + + n(n + 2) = n/6 (n + 1)(2n + 7)
-
How do you manage global and international teams? What would you do different?
-
Your friend is super excited about the results of their study! They examined whether different parenting styles [A] resulted in differences in anxiety levels among children. The different levels (a)...
-
Test the series for convergence or divergence. n=1 e1/n 78 O convergent O divergent
-
How do employees perceive the organization's vision and mission, and to what extent do these perceptions influence their commitment to the organization ?
-
In the table below which shows class taken and grade achieved, find the probability that a student selected takes Stat or receives a B grade. Round your answer to three decimal places 40 70 70 50 40...
-
Describe the different types of impasse-resolution procedures used in the public sector, and discuss the relative effectiveness of each?
-
An environmentalist wants to determine if the median amount of potassium (mg/L) in rainwater in Lincoln County, Nebraska, is different from that in the rainwater in Clarendon County, South Carolina....
-
Predict the product of the Dieckmann cyclization that occurs when each of the following compounds is treated with sodium ethoxide. (a) (b) (c) OEt LOET Eto OEt
-
When the following compound is treated with sodium ethoxide, two condensation products are obtained, both of which are produced via Dieckmann cyclizations. Draw both products. OEt Eto
-
For each of the following reactions, predict the major product and propose a mechanism for its formation. (a) (b) (c) :? 1) LDA 2) CH3I 1) NaH 2) -CH,Br
-
please answer 2&3. Thanks! 2. The following information was taken from the financial records for Whitlock Industries for the years 2019 and 2020. The balance sheet items were recorded at the end of...
-
Alex and Bess have been in partnership for many years. The partners, who share profits and losses on a 60:40 basis, respectively, wish to retire and have agreed to liquidate the business. Liquidation...
-
Suppose a company estimates the following for a product it sells: Tangible value the product provides: $ 2 5 Intangible value the product provides: $ 1 5 Costs customer must incur to purchase the...

Study smarter with the SolutionInn App


