When the following compound is treated with sodium ethoxide, two condensation products are obtained, both of which
Question:
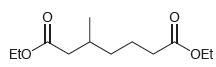
Transcribed Image Text:
OEt Eto
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When the following compound is treated with sodium ethoxide, nearly all of it is converted into an enolate. Draw the resonance structures of the enolate that is formed, and explain why enolate...
-
What product is formed when the following compound is treated with Ag2O? HO
-
When the following compound is treated with Br 2 in the presence of a Lewis acid, one product predominates. Determine the structure of that product. Br FeBr3 ?
-
find the steady state expression for vo in the circuit fig 9.32 if ig = 500cos2000tmA 3) 9.32 Find the steady-state expression for u,, in the circuit of Fig. P9.32 if i = 500 cos 2000 mA. Figure...
-
Amy is 12 years old now and will attend college at age 18. Her parents plan to fund her college for four years. College costs $20,000 per year as of the time when Amy turns to age 18. If her parents...
-
Solve each system. If a system is inconsistent or has dependent equations, say so. 3x + 5y + 3z = 2 6x + 5y + z = 0 3x + 10y - 2z = 6 =
-
In the section on the yield to call, a bond pays annual interest of $80 and matures after ten years. The bond is valued at $1,147 if the comparable rate is 6 percent and the bond is held to maturity....
-
Five mutually exclusive revenue alternatives that have infinite live s are under consideration for increasing productivity in a manufacturing operation. The initial costs and cash flows of each...
-
Why is it necessary to consider the economic and demographic environment in assessing a governments financial condition? How is the budgetary cushion calculated, and why should a government maintain...
-
Gaines Company recently initiated a postaudit program. To motivate employees to take the program seriously, Gaines established a bonus program. Managers receive a bonus equal to 10 percent of the...
-
Ask students to list three products that seem to have personalities. Describe the personalities. What types of people buy these products? Is there a match between the consumers personality and that...
-
This chapter mentions that psychographic analyses can be used by politicians to market themselves. What are some of the marketing strategies and techniques used by politicians in recent elections?...
-
Calculate the emission wavelength (nm) of excited atoms that lie 3.371 10-19 J per molecule above the ground state.
-
Determine the magnitude of the magnetic flux through the south-facing window of a house in British Columbia, where Earth's B field has a magnitude of 5.8 x 10-5T and the direction of B field is 72...
-
A wedge with an inclination of angle rests next to a wall. A block of mass m is sliding down the plane, as shown. There is no friction between the wedge and the block or between the wedge and the...
-
Conner Leonard worked for Purges Manufacturing for 32 years. Along with four other men, he helped to start the company that designed and built products sold around the world. Purges Manufacturing...
-
Reconsider the collision between two objects diagrammed below where two objects move on a frictionless surface. Before collision After collision Experiment 1 A, 1 B A B Draw complete and properly...
-
3. Now the bomb arrives. Please catch fx,y(x, y) = = cx cx - dy, where 0 < x < 1, 0 y x. 13 a) Please find coefficients c, d such that cd= 8 b) Please find fx(x) and fy (y). Are X and Y independent?...
-
Sand is leaking from a bag in such a way that after t seconds, there are pounds of sand left in the bag. a. How much sand was originally in the bag? b. At what rate is sand leaking from the bag after...
-
All of the following assets can be depreciated, except: (a) A bulldozer (b) A copper mine (c) A surgical robot (d) A conveyor belt
-
How many milliliters of aqueous 0.1 M NaOH are required to form the disodium salt from 100 mg of succinic acid?
-
Give the product(s) formed and the curved-arrow notation for the reaction of 0.01 mole of each reagent below with 0.01 mole of acetic acid. (a) Cs+ -OH (b) H3C--Li (c) NaH
-
Explain why the differences between the first and second pKa values of the dicarboxylic acids become smaller as the lengths of their carbon chains increase (Table 20.3). TABLE 20.3 pK, Values of Some...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


