What product is formed when the following compound is treated with Ag2O? HO
Question:
What product is formed when the following compound is treated with Ag2O?
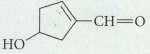
Transcribed Image Text:
HO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
Silver I oxide oxidizes t...View the full answer

Answered By

Vikas NA
I have 1 year teaching experience in various field such as mathematics, computer science, chemistry. Mathematics is my most interesting subject. I have work as a private tutor in aakash institute for mathematics subject for 0.5 years. then, i was move to other coaching center. I was work in Surya shiksha mandir coaching center as a chemistry tutor for 2 months. After that, i joined coding blocks institute as a computer science tutor and i teach c++, java, python, matlab, database, operating system. I worked 5 month in this institute. My overall teaching experience is very good, student give me very good reviews.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
When the following compound is treated with sodium ethoxide, two condensation products are obtained, both of which are produced via Dieckmann cyclizations. Draw both products. OEt Eto
-
When the following compound is treated with concentrated HCl at 100ºC for several hours, hydrolysis occurs, producing one of the 20 naturally occurring amino acids. Identify which one. H,N,
-
When the following compound is treated with excess methyl iodide, a quaternary ammonium salt is obtained that bears only one positive charge. Draw the structure of the quaternary ammonium salt. *NH2
-
Ag Bio Tech (ABT) was organized on January 1, 2013, by four friends. Each organizer invested $10,000 in the company and, in turn, was issued 8,000 shares of common stock. To date, they are the only...
-
1. A foundation pledges to donate $1 million to an art institute one year in the future. When, and in what amount, should the institute recognize revenue? The institute applies a discount rate of 10...
-
How much SSI is paid by a person with an annual income of \(\$ 183,500\) ?
-
Using the PewKids dataset, run a frequency distribution (and get descriptive statistics) on the variable for the number of texts a teenager sends per day. Should the kids who replied 500 or more be...
-
The El Dorado Star is the only newspaper in El Dorado, New Mexico. Certainly, the Star competes with The Wall Street Journal, USA Today, and the New York Times for national news reporting, but the...
-
following costs of making the part: contribution margin per unit of $ 7 . 5 0 . What is the financlal advantage ( disadvantage ) of buying 1 0 , 0 0 0 units from the supplier? Multiple Choice $ ( 6 0...
-
Please look at the following graph and answer questions. If a node has more than one neighbor, visit the neighbors in alphabetical order. For example, when you iterate over the neighbors {A, F, D, Z}...
-
Draw the structures of all aldehydes or ketones that could in principle give the following product after application of either the Wolff-Kishner or Clemmensen reduction. CH,CH(CH)h
-
Give the product expected (if any) when butyraldehyde (butanal) reacts with each of the following reagents. (a) PhMgBr, then dilute H3O+ (b) LiAlH4 in ether, then H3O+ (c) Alkaline KMnO4, then H3O+...
-
On September 1 of the current year, Joy Tucker established a business to manage rental property. She completed the following transactions during September: A. Opened a business bank account with a...
-
My first run at a dissertation was on Dr. Martin Luther King, Jr. When I was very young he walked through my hometown of Albany, Georgia. My father accompanied him, more to protect him than anything,...
-
Question 2 are charged, and the charge on sphere Y is The X and Y dots shown in the figure are two identical spheres, X and Y, that are fixed in place with their centers in the plane of the page....
-
how do i get the residuel income please help in just need the cell formula in excel 2 Genmure Corporation is trying to analyze the results of three efficiency initiatives that were taken on the...
-
Harlow Appliance has just developed a new air fryer it believes will have broad market appeal. The company has performed marketing and cost studies that revealed the below information: a. New...
-
Based on the business that you created a global strategy for in the week 4 discussion, determine a low-cost & differentiation strategy in an effort to remain competitive in the global market. Include...
-
How many, and which, factors determine portfolio risk?
-
The following selected accounts and normal balances existed at year-end. Notice that expenses exceed revenue in this period. Make the four journal entries required to close the books: Accounts...
-
Use the information in Figure 4.2 to predict the positions of the equilibria in the reactions in problem 4.4.
-
Draw diagrams like that in Figure 4.3 for the reactions in problem 4.9.
-
Show a free energy versus reaction progress diagram for the following reaction: HCI+ NH3 CI + NH4
-
A family has a $117,443, 25-year mortgage at 5.4% compounded monthly. (A) Find the monthly payment and the total interest paid. (B) Suppose the family decides to add an extra $100 to its mortgage...
-
Comparing the actual and planned cost of a consulting engagement completed by an engineering firm such as Allied Engineering.
-
What is the NPV of a project that costs $34,000 today and is expected to generate annual cash inflows of $11,000 for the next 7 years, followed by a final inflow of $14,000 in year 8. Cost of capital...

Study smarter with the SolutionInn App


