When the following compound is treated with concentrated HCl at 100ºC for several hours, hydrolysis occurs, producing
Question:
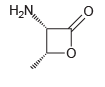
Transcribed Image Text:
H,N,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
HN HCI H...View the full answer

Answered By

Vikash Gupta
I am graduated in Physics in 2018, from KIRORIMAL COLLEGE, University of Delhi. Now I am persuing Master's degree in physics. I like to do physics problems. I have experience of 1 year in tutoring. I think Physics is the only subject where you understand things,how they are happening . In physics you learn Maths and apply it. So I would like to join your platform to solve many Physics problems.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What product is formed when the following compound is treated with Ag2O? HO
-
When the following compound is treated with sodium methoxide in methanol, two elimination products are possible. Explain why the deuterated product predominates by about a 7:1 ratio (refer to Problem...
-
Only a substitution product is obtained when the following compound is treated with sodium methoxide: Explain why an elimination product is not obtained. CH3 Br CH3
-
6. Kindly describe the accounting framework. What are the elements and how do they effect the financial statements? 7. What does the accounting model specify? What are the elements involved?
-
The Finishing Department of Lee and Lewis, Inc., the last department in the manufacturing process, incurred production costs of $220,000 during the month of June. If the June 1 balance in...
-
What is an equipotential line? What is an equipotential surface?
-
Write notes on (a) Normal loss (b) Abnormal loss (c) Abnormal gain
-
(a) Using the Consolidated Balance Sheets for Walgreen Co. for August 31, 2013 and 2012, prepare a common-size balance sheet. (b) Which current asset is the most significant? Which noncurrent asset...
-
How long does it take to double your investment portfolio if it earns an annual return of 5 % .
-
Tobias Eaden started a sole proprietorship named Sky High Ads. A customers message can be displayed on an airplane banner across the city. The following are Sky High Ads business activities during...
-
We saw in Section 25.6 that DCC can be used to form a peptide bond. We explored the mechanism, and we saw that DCC activates the COOH moiety so that it readily undergoes nucleophilic acyl...
-
Waxes can be hydrolyzed to yield an alcohol and a carboxylic acid. Draw the products obtained when triacontyl hexadecanoate undergoes hydrolysis.
-
In a column in the Washington Post, Robert J. Samuelson wrote: "As for what's caused greater inequality, we're also in the dark. The Reagan and Bush tax cuts are weak explanations, because gains have...
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
Evaluate the following integrals. 4 IST Jo Jo x dz dx dy
-
Which of the following raises the credibility of areport? Which of the following raises the credibility of a report? Multiple Choice avoiding predictions avoiding the use of cause-effect statements...
-
Addition of 1-bromobut-2-ene to magnesium metal in dry ether results in formation of a Grignard reagent. Addition of water to this Grignard reagent gives a mixture of but-1-ene and but-2-ene (cis and...
-
Show how you might synthesize the following compounds starting with alkyl, alkenyl, or aryl halides containing four carbon atoms or fewer. (a) 3-phenylprop-1-ene (b) 5-methylhex-2-ene c) dec-5-ene
-
Predict the products of the following proposed Diels-Alder reactions. (a) (b) (c) (d) (e) (f) CHO C-C-IC-C CN NC CN O + OCHs CN CH,O CN
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...

Study smarter with the SolutionInn App


