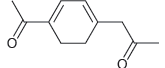
When the following compound is treated with sodium ethoxide, nearly all of it is converted into an
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
This anion is highly stabilized b...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When the following compound is treated with sodium ethoxide, two condensation products are obtained, both of which are produced via Dieckmann cyclizations. Draw both products. OEt Eto
-
Only a substitution product is obtained when the following compound is treated with sodium methoxide: Explain why an elimination product is not obtained. CH3 Br CH3
-
When the following compound is treated with sodium methoxide in methanol, two elimination products are possible. Explain why the deuterated product predominates by about a 7:1 ratio (refer to Problem...
-
Prove that point B in Figure 18.1 is not Pareto effi cient. Food Fo FB OA Abner E C B Contract curve UAB UBB MB -Medicine Z Belinda Mo Figure 18.1 Edgeworth Box for Exchange; Pareto Efficient Points...
-
Describe the current situation at Bayonne. How might Milliken summarize Bayonne's performance problems?
-
Find the remaining roots of the given equation using synthetic division, given the roots indicated. 4x + 6x 2x 1 = 0 (r 1 = 1/2)
-
In cost plus contracts, the contractor will get cost plus a stipulated profit.
-
A manufacturing company employs several maintenance employees. When a problem occurs with the equipment, a maintenance employee receives a description of the symptoms and is supposed to locate and...
-
Walton Manufacturing Co. produces and sells specialized equipment used in the petroleum industry. The company is organized into three separate operating branches: Division A, which manufactures and...
-
An analysis of transactions for Arthur Cooper & Co. was presented in Exercise. In Exercise, An analysis of the transactions made by Arthur Cooper & Co., a certified public accounting firm, for the...
-
How have automated work flows tended to impact supply chains?
-
Describe the customer contact points of a favorite restaurant, and discuss whether there should be fewer or more contact points.
-
Find the most general antiderivative of the function. (Check your answer by differentiation.) f(x) = x 2 3x + 2
-
The balances of selected accounts of Casper Company on February 28, 20X1, were as follows: Sales $250,000 and Sales Returns and Allowances $4,000. The firm's net sales are subject to an 7 percent...
-
1. Draw and label force diagrams for the physics book and for the calculator. Add equality marks showing any equalities between force diagrams. Circle and label any Newton's third law pairs. (6 pts)...
-
Consider the Lincoln Tunnel, which was built in 1939 under the Hudson River in New York. Assume the tunnel to be empty with perfectly conducting walls and rectangular cross section with width 6.55 m...
-
Examine a well-known principal-agent contract, the sale of your home by a licensed realtor. You will use the following data to analyze this case. Your home is the typical home, approximately 1,875 sq...
-
i) Generate a third degree polynomial in x and y named g(x, y) that is based on your mobile number (Note: In case there is a 0 in one of the digits replace it by 3). Suppose your mobile number is...
-
In Problems 5772, list the intercepts and test for symmetry. y = x + 4
-
A horizontal annulus with inside and outside diameters of 8 and 10 cm, respectively, contains liquid water. The inside and outside surfaces are maintained at 40 and 20oC, respectively. Calculate the...
-
Propose a curved-arrow mechanism for each of the re-actions given in Fig. P17.38. (a) (b) (c) H ,C) ether CH:(CH2)3 CH C CH2 CH3(CH2sC C CH 1-penten-4-yne + Na+:C CH; then allyl bromide- H,C-CH-CH-C...
-
What product(s) would be expected in the same re-action of 3-methyl-4-octyne? Explain.
-
Propose a curved-arrow mechanism for the following reaction. Explain why the equilibrium lies to the right. Ph Ph CH toluenesulfonic acidCH
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


