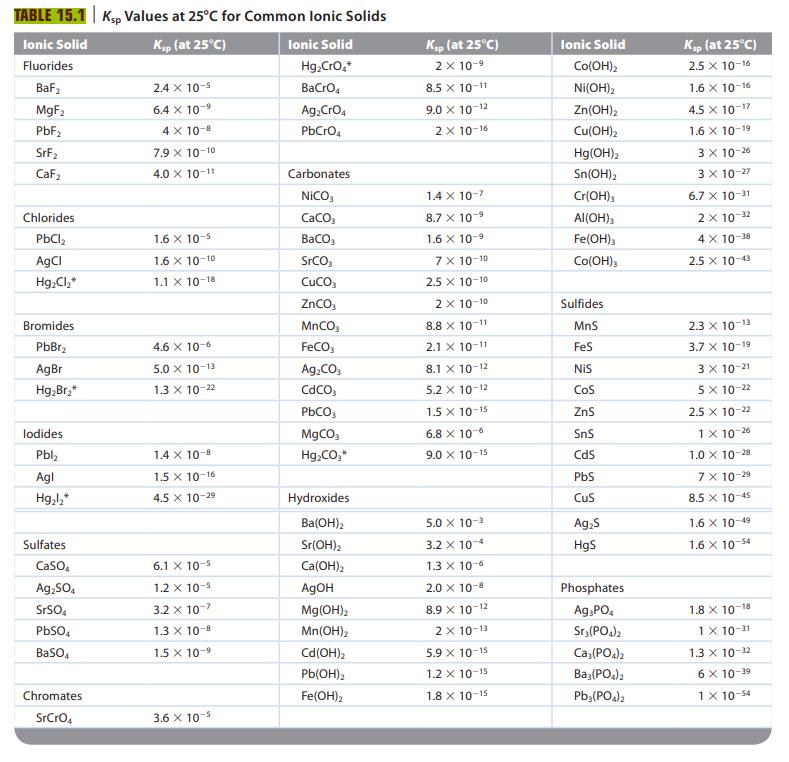
To what reaction does the solubility product constant, Ksp, refer? Table 15.1 lists K sp values for
Question:
To what reaction does the solubility product constant, Ksp, refer? Table 15.1 lists Ksp values for several ionic solids. For any of these ionic compounds, you should be able to calculate the solubility. What is the solubility of a salt, and what procedures do you follow to calculate the solubility of a salt? How would you calculate the Ksp value for a salt given the solubility?
Transcribed Image Text:
TABLE 15.1 Ksp Values at 25°C for Common Ionic Solids Ionic Solid Ksp (at 25°C) Fluorides BaF₂ MgF₂ PbF₂ SrF₂ CaF₂ Chlorides PbCl₂ AgCl Hg₂Cl₂* Bromides PbBr₂ AgBr Hg₂Br₂* lodides Pbl₂ Agl Hg₂l₂* Sulfates CaSO4 Ag₂SO4 SrSO4 PbSO4 BaSO4 Chromates SrCrO4 2.4 x 10-5 6.4 x 10-9 4 X 10-8 7.9 X 10-10 4.0 X 10-11 1.6 X 10-5 1.6 X 10-10 1.1 X 10-18 4.6 x 10-6 5.0 X 10-13 1.3 x 10-22 1.4 x 10-8 1.5 X 10-16 4.5 X 10-2⁹ 6.1 x 10-5 1.2 x 10-5 3.2 x 10-7 1.3 X 10-8 1.5 X 10-9 3.6 x 10-5 Ionic Solid Hg₂CrO,* BaCrO4 Ag₂ CrO4 PbCrO4 Carbonates NICO, CaCO3 BaCO3 SrCO CUCO3 ZnCO₂ MnCO₂ FeCO3 Ag₂CO3 CdCO PbCO3 MgCO3 Hg₂CO,* Hydroxides Ba(OH)2 Sr(OH)2 Ca(OH)2 AgOH Mg(OH)2 Mn(OH)₂ Cd(OH)₂ Pb(OH)2 Fe(OH)₂ Ksp (at 25°C) 2 x 10-⁹ 8.5 X 10-11 9.0 X 10-12 2 X 10-16 1.4 x 10-7 8.7 X 10 -9 1.6 × 10-9 7 x 10-10 2.5 X 10-10 2 x 10-10 8.8 x 10-11 2.1 X 10-11 8.1 X 10-12 5.2 x 10-12 1.5 X 10-15 6.8 x 10-6 9.0 X 10-15 5.0 × 10-³ 3.2 x 10-4 1.3 x 10-6 2.0 × 10-8 8.9 X 10-12 2x 10-13 5.9 X 10-15 1.2 X 10-15 1.8 X 10-15 Ionic Solid Co(OH)2 Ni(OH)2 Zn(OH)₂ Cu(OH)₂ Hg(OH)2 Sn(OH)2 Cr(OH)3 Al(OH)3 Fe(OH)3 Co(OH)3 Sulfides MnS FeS NIS COS ZnS SnS CdS PbS CuS Ag₂S HgS Phosphates Ag3PO4 Sr3(PO4)2 Ca3(PO4)2 Ba3(PO4)2 Pb3(PO4)2 Ksp (at 25°C) 2.5 X 10-16 1.6 X 10-16 4.5 X 10-1 1.6 X 10-19 3 x 10-26 3 x 10-27 6.7 X 10-31 2 x 10-32 4 X 10-38 2.5 X 10-43 2.3 X 10-13 3.7 X 10-19 3 x 10-21 5 x 10-22 2.5 x 10-22 1 X 10-26 1.0 X 10-28 7 x 10-29 8.5 x 10-45 1.6 X 10-49 1.6 x 10-54 1.8 X 10-18 1 X 10-31 1.3 x 10-32 6 x 10-39 1 x 10-54
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Lets figure out how soluble BaCrO 4 is Utilizing the supplied K sp value from Table 151 which i...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Write a project management plan. we have a template and project description. we need to edit the template(table of contents) with our own ideas. CPSC 8820-01 Project Management Plan Your Unique...
-
Read the article " The Twitter Trap " by Bill Keller carefully. In this article, Keller uses his personal experiences to attract the audience and uses funny examples to help the audience understand...
-
Calculate the solubility product constant for Mg(OH)2 at 25oC. The Gof values (in kJ/mol) are as follows: Mg2+ (aq), 454.8; OH(aq), 157.3; Mg(OH)2(s), 833.7.
-
State whether or not each of the following events would result in a liability being recognised in the accounts at 30 June. 1. Taxes for the year ended 30 June, which are not payable until October. 2....
-
Go to this texts Web site at academic. cengage.com/blaw/clarkson and select Chapter 49. Click on Video Questions and view the video titled Double Indemnity. Then answer the following questions. (a)...
-
A tank of gas at 21°C has a pressure of 1.0 atm. Using the data in the table, answer the following questions. Explain your answers. a. If the tank contains carbon tetrafluoride, CF4, is the...
-
How important do you think Cloughertys experience as a referee was in preparing him to be a supervisor? Other than that work experience, what experiences and qualities do you think would be important...
-
Veekay Company was organized on November 1 of the previous year. After seven months of start-up losses, management had expected to earn a profit during June, the most recent month. Management was...
-
QUESTION 4 (please complete both parts A and B) 4 PART A The following information has been extracted from the accounts of Hawthorn Pty Ltd. (a resident private company) for the year ended 30 June...
-
Darius Williams, 44, is a single father with three-year old twin boys, who just lost his job as a Finance Analyst. He was employed for nine (9) years with Vroom Finance, a vehicle financing company....
-
Fig. 15.2 summarizes the classic method for separating a mixture of common cations by selective precipitation. Explain the chemistry involved with each of the four steps in the diagram. Fig. 15.2...
-
Consider the following four titrations (iiv): a. Rank the four titrations in order of increasing pH at the halfway point to equivalence (lowest to highest pH). b. Rank the four titrations in order of...
-
What other peripheral devices and capabilities would you want to have for your business PC? Explain your choices.
-
Salinger Company estimates that total factory overhead costs will be $70,000 for the year. Direct labor hours are estimated to be 10,000. a. For Salinger Company, determine the predetermined factory...
-
SCS receives on average 1 data package every 1/50 seconds, with a standard deviation of 1/50 seconds, and processes them using its single powerful computing unit, which can process data packages in...
-
Suppose that we pay workers $25 per day.We value processed orders at $4 per order and the number of orders each worker can process is worker 1 - 8 orders, worker 2 - 7 orders, worker 3 - 6 orders,...
-
How do I imagine that I am the administrator of a midsize long-term care facility with an outdated information system and I have been given thetaskto planand managethe integration of a new database...
-
You are negotiating a five - year contract with a new customer. The contract could be larger than any previous contracts your company has had. Which would be your best negotiation style?
-
Explain why some states do and other states don't have a public sector bargaining law or laws that cover some public employees but not others?
-
Which of the following is NOT a magnetic dipole when viewed from far away? a) A permanent bar magnet. b) Several circular loops of wire closely stacked together with the same current running in each...
-
Identify the reagents you would use to achieve the following transformation:
-
Propose an efficient synthesis for each of the following compounds using the malonic ester synthesis. (a) (b) (c) (d) (e)
-
Starting with diethyl malonate and using any other reagents of your choice, proposean efficient synthesis for each of the following compounds: (a) (b) (c) .
-
Whispering Winds Corporation is authorized to issue 22,500 shares of $50 par value, 10% preferred stock and 130,000 shares of $5 par value common stock. On January 1, 2022, the ledger contained the...
-
Question Help Runaround Corporation sells running shoes and during January they ran production machines for 22,000 hours total and incurred $10,000 in maintenance costs. During July they ran...
-
Exercise 8-3 (Algo) Preparing flexible budgets LO P1 Tempo Company's fixed budget (based on sales of 16,000 units) folllows. 3,280,000 Fixed Budget Sales (16,000 units X $205 per unit) Costs Direct...

Study smarter with the SolutionInn App


