Starting with diethyl malonate and using any other reagents of your choice, proposean efficient synthesis for each
Question:
(a)
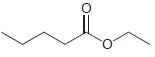
(b)
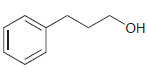
(c)
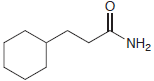
Transcribed Image Text:
но.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
a b c EtO O...View the full answer

Answered By

Junaid ahmed
I am an English language professor with years of experience In Teaching English Language and Literature. I like to help people in the various difficult matter.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Starting with diethyl malonate, and using any other reagents of your choice, show how you would prepare each of the following compounds. (a) (b) (c)
-
Starting with cyclohexene and using any other reagents of your choice, show how you would prepare each of the following compounds. a. b. c. OH OMe
-
Starting with cyclopentanone and using any other reagents of your choosing, identify how you would prepare each of the following compounds: (a) (b) (c) (d)
-
What is the purpose of a Notice of Assessment?
-
Jorge Cabrera paid $980 for a 15-year bond 10 years ago. The bond pays a coupon of 10 percent semiannually. Today, the bond is priced at $1,054.36. If he sold the bond today, what would be his...
-
The Wadena Company reports the following information pertaining to the month of January: During January, the company purchased $40,000 of direct materials and incurred $90,000 of direct labor costs....
-
What information must a company disclose about its products and services and about its operations in foreign countries? LO3
-
It seems as if consolidated net income is always less than the sum of the parent's and subsidiary's separately calculated net incomes. Is it possible that the consolidated net income of the two...
-
Consider a bond selling at par with modified duration of 12 years and convexity of 265. A 1% decrease in yield would cause the price to increase by 12%, according to the duration rule. What would be...
-
1. Write a brief report that outlines the reasons (both internal and external) for Burgmasters demise, and whether operations management played a significant role in the demise. 2. Do you think that...
-
Propose an efficient synthesis for each of the following compounds using the malonic ester synthesis. (a) (b) (c) (d) (e)
-
Consider the illustrative example of Section 10.6 (Example 10.1). How would you reconcile the difference in the marginal propensity to consume obtained from Eqs. (10.6.1) and (10.6.4)?
-
You would like to ensure that your sample is representative of the racial mix seen in your population of interest. The population is 50% Asian, 20% African American, 20% Caucasian, and 10% other. You...
-
Context This task requires analysing a network scenario, design the network architecture and recommend IT solutions including ethical, security and sustainability considerations.The purpose of this...
-
What was the Prime Cost Percent for Mandy's BBQ Pit for August? Select one: a. 46.5% b. 73.9% c. 63.4% d. 85%
-
Finding Critical Values and Confidence Intervals. In Exercises 5-8, use the given information to find the number of degrees of freedom, the critical values x? and x*, and the confidence interval...
-
An investor sold 100 shares of ABC stock short at $25 and buys one ABC Jan 30 call @ $5. What is this investor's maximum gain, maximum loss, and breakeven points from this strategy?
-
Jake, Sachs and Brianne own a tour company called Adventure Sports. The partners share profits and losses in a 1:3:4 ratio. After Lengthy Dissagreements among the partners and several unprofitable...
-
In Exercises 61 through 64, the position s(t) of an object moving along a straight line is given. In each case: (a) Find the objects velocity v(t) and acceleration a(t). (b) Find all times t when the...
-
The maximum pressure that can be developed for a certain fluid power cylinder is 15.0 MPa. Compute the required diameter for the piston if the cylinder must exert a force of 30 kN.
-
How many products are formed when compound B is decarboxylated? , -
-
Given that squaric acid behaves like a dicarboxylic acid, draw structures for the products formed when it reacts with excess SOCl2; with ethanol solvent in the presence of an acid catalyst.
-
Complete each of the reactions given in Fig. P20.49 by giving the principal organic product(s). Give the reasons for your answers. H,C KOH PhCH Cl acid HO,C CO,H+ethylene glycolheolym) CH + Hg(OAch...
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


