For the following, mix equal volumes of one solution from Group I with one solution from Group
Question:
For the following, mix equal volumes of one solution from Group I with one solution from Group II to achieve the indicated pH. Calculate the pH of each solution.
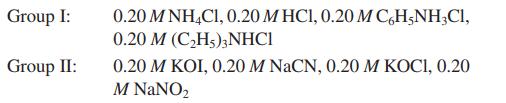
a. The solution with the lowest pH
b. The solution with the highest pH
c. The solution with the pH closest to 7.00
Transcribed Image Text:
Group I: Group II: 0.20 M NH4C1, 0.20 M HC1, 0.20 M C6H5NH₂Cl, 0.20 M (C₂H5)3NHC1 0.20 M KOI, 0.20 M NaCN, 0.20 M KOCI, 0.20 M NaNO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a The solution with the lowest pH The solution with the lowest pH will be the one formed by mixing NH4Cl from Group I with HCl from Group II When NH4C...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
What error in this program needs to be fixed? def sum(num1, num2): return num1 + num2 5.5, 6)) print(sum(4,
-
Consider the pooled t variable Tp from part (b) of the previous exercise. a. Use this t variable to obtain a pooled t confidence interval formula for 1 2 . b. The article Effect of Welding on a...
-
If there was any ambiguity on the application, should it be resolved in favor of the insured or the insurer? Provident Insurance, Inc., issued an insurance policy to a company providing an employee,...
-
Consider the following potential-energy curves for two different reactions: a. Which reaction has a higher activation energy for the forward reaction? b. If both reactions were run at the same...
-
Do you consider yourself a highly ambitious person? Yes / No
-
The Bee Line Caf is well known for its popular homemade ice cream, which it makes in a small plant in back of the cafe. People drive long distances to buy the ice cream. The two ladies who own the...
-
Will rate thumbs up! Caspian Sea Drinks is considering the production of a diet drink. The expansion of the plant and the purchase of the equipment necessary to produce the diet drink will cost...
-
Figure 4-32 shows a class list for Millennium College. Convert this user view to a set of 3NF relations using an enterprise key. Assume the following: ¢ An instructor has a unique location....
-
A 0.100-g sample of the weak acid HA (molar mass = 100.0 g/mol) is dissolved in 500.0 g water. The freezing point of the resulting solution is -0.0056C. Calculate the value of K a for this acid....
-
Calculate the pH of a 0.10-M solution of sodium phosphate. (See Exercise 183.) Data in Exercise 183 Consider the species PO 4 3- , HPO 4 2- , and H 2 PO 4 - . Each ion can act as a base in water....
-
Financial reporting classifies derivatives as (a) speculative investments, (b) fair value hedges, or (c) cash flow hedges. However, firms revalue all derivatives to market value each period...
-
How do cognitive biases such as confirmation bias, anchoring, and the availability heuristic influence the quality of decision-making within complex organizational contexts ?
-
What role do cognitive biases, such as confirmation bias and anchoring, play in perpetuating conflict, and how can awareness of these biases facilitate more effective conflict resolution strategies?
-
Were you surprised by the results? Do you agree with the results? How can you use this knowledge of your personal biases to inform your management strategies? How can the identified biases impact...
-
what ways do existing power structures perpetuate social stratification, and what are the socio-political ramifications of these dynamics ?
-
How do feedback loops and reflective practices contribute to continuous improvement and the refinement of teamwork dynamics over time ? Explain
-
The Steelworkers' Trilogy greatly enhanced the arbitrator's authority when compared to previous years, yet did not give the arbitrator final jurisdiction over certain issues. Discuss the preceding...
-
From a medical tourist perspective, compare Shouldice with the traditional hospital in terms of the key factors of competition. Using Table 15-3, why would Shouldice attract patients from outside the...
-
Diethyl malonate (the starting material for the malonic ester synthesis) reacts with bromine in acid-catalyzed conditions to form a product with molecular formula C 7 H 11 BrO 4 . (a) Draw the...
-
Cinnamaldehyde is one of the primary constituents of cinnamon oil and contributes significantly to the odor of cinnamon. Starting with benzaldehyde and using any other necessary reagents, show how...
-
Draw the condensation product that is expected when each of the following esters is treated with sodium ethoxide followed by acid workup. (a) (b) (c) OEt OEt
-
On September 18, 2019, Rose Company purchased 11,800 shares (14%) of Wozniak, Inc. stock for $42 per share. The market value of the Wozniak stock at December 31, 2019 was $29 per share. Rose Company...
-
Determine the standard direct labor hours per tune-up. (Round answer to 2 decimal places, e.g. 15.10.) Standard direct labor hours per tune-up hours (b) Determine the standard direct labor hourly...
-
The registration advisors at a small midwestern university (SMU) help 6,000 students develop their class schedules and register for classes each semester. Each advisor works for 10 hours a day during...

Study smarter with the SolutionInn App


