Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write
Question:
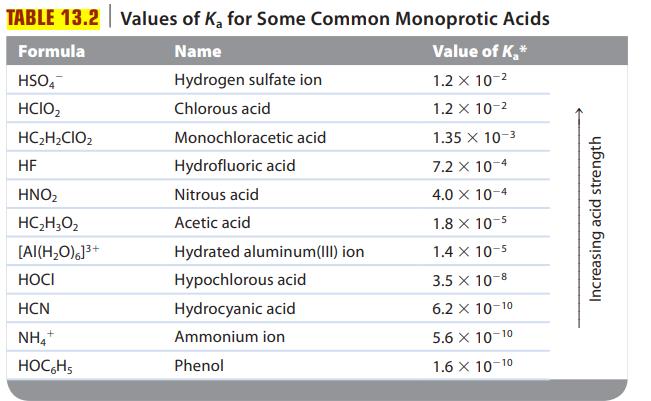
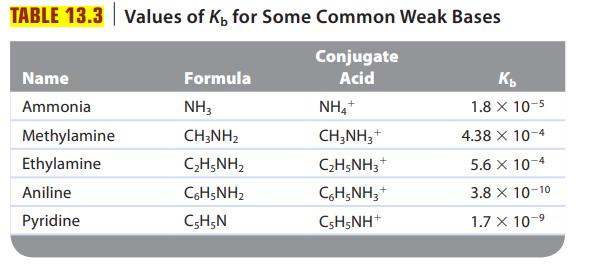
Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka and Kb values are found in Tables 13.2 and 13.3.
Transcribed Image Text:
TABLE 13.2 Values of K₂ for Some Common Monoprotic Acids Name Value of K₂* Hydrogen sulfate ion 1.2 x 10-² Chlorous acid 1.2 x 10-² 1.35 x 10-3 7.2 x 10-4 4.0 X 10-4 Formula HSO4 HCIO₂ HC₂H₂CIO₂ HF HNO₂ HC,H,Oz [AI(H₂O)]³+ HOCI HCN NH4+ HOCHS Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol 1.8 x 10-5 1.4 x 10-5 3.5 x 10-8 6.2 X 10-10 5.6 X 10-10 1.6 X 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
Solutions of the following salts are acidic basic or neutral Salt Solution Equation NaHS Basic NaHS H2O HS Na H3O NH4Cl Acidic NH4Cl NH4 Cl NaCH3COO B...View the full answer

Answered By

Joemar Canciller
I teach mathematics to students because I love to share what I have in this field.
I also want to see the students to love math and be fearless in this field.
I've been tutoring these past 2 years and I would like to continue what I've been doing.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Lets take a look at the extensive career of Helen Frankenthaler (1928-2011), who found a way to incorporate all of the innovative techniques artists were experimenting with starting in 1945. Her...
-
Will 0.10 M solutions of the following salts be acidic, basic, or neutral? a. Ammonium bicarbonate b. Sodium dihydrogen phosphate c. Sodium hydrogen phosphate d. Ammonium dihydrogen phosphate e....
-
Decide whether solutions of the following salts are acidic, neutral, or basic. a. Ammonium acetate b. Anilinium acetate
-
Which of the following would be the most frequently occurring daily transaction in a retail shop? (a) Paying salary to the sales assistant (b) Sale of goods (c) Payment of rent for the shop premises...
-
James Lillards first wife had a child whom James adopted when he married that childs mother. James fathered other children with her until they divorced in the early 1970s. In 1975, James married his...
-
What is the equilibrium composition of a reaction mixture if you start with 0.500 mol each of H2 and I2 in a 1.0-L vessel? The reaction is H2(g) + 12(g)--2HI(g) Kc = 49.7 at 458C
-
Explain how supervisors can develop and maintain good relations with their employees, managers, and peers.
-
The following statement was made by the vice president of finance of Orville Inc.: The managers of a company should use the same information as the shareholders of the firm. When managers use the...
-
A tourist traveling with $10,000 in cash. At the hotel, he is informed that he has a one in two chance of being robbed during the night. The hotel has a safe, which costs $ 4000. Hence the tourist is...
-
"Part 1: The Performance Lawn Equipment database contains data needed to develop a pro forma income statement. Dealers selling PLE products all receive 18% of sales revenue for their part of doing...
-
Consider a solution of an unknown salt having the general formula BHCl, where B is one of the weak bases in Table 13.3. A 0.10-M solution of the unknown salt has a pH of 5.82. What is the actual...
-
The K b values for ammonia and methylamine are 1.8 10 -5 and 4.4 10 -4 , respectively. Which is the stronger acid, NH 4 + or CH 3 NH 3 + ?
-
There are two basic accounting approaches to reporting accounting changes. What are they?
-
Why is it critical to immediately contact your Engagement Partner when you suspect or identify non-compliance? He or she will ensure that the non-compliance doesn't affect the Client's reputation He...
-
Question 9: Determine the current and its direction, in each resistor, for the circuit shown below. Show your calculations. R=152 9.0 V + 12V ww R=75 2 R3= 50
-
how can The High - Tech Way To Recycle Clothes sustainable. and what they offer and what are their ecofriendly
-
James Bondbuyer purchases a Treasury bond on Monday, May 2, regular way settlement. The bond pays interest on January 15 and July 15. How many days of accrued interest will be owed to the seller? A...
-
Aviation and air traffic control have come a long way in the last 100-years. Some believethat we have reached a plateau and that growth in aviation will stop. Aviation may go the way of the railroads...
-
If you were a secondary employer confronted with a threatened product picket action at your retail store, what factors would you consider in deciding whether to voluntarily cease sales or continue to...
-
Dan and Diana file a joint return. Dan earned $31,000 during the year before losing his job. Diana received Social Security benefits of $5,000. a. Determine the taxable portion of the Social Security...
-
Draw the structure of the product with molecular formula C 10 H 10 O that is obtained when the compound below is heated with aqueous acid. CN CN C10H100 Heat
-
Lactones can be prepared from diethyl malonate and epoxides. Diethyl malonate is treated with a base, followed by an epoxide, followed by heating in aqueous acid: Using this process, identify what...
-
Predict the major product of the following transformation. CO2ET C10H100 Heat
-
The Lenzie Corporation's common stock has a beta of 1.15. If the risk free rate is 3.5% and the expected retum on the market is what is the company's cost of equity capital? (Do hot round...
-
PnR Catering Ltd.is a limited company (the Company) which engages in the airline catering services and owns a multi-storey building next to the Hong Kong International Airport, where all the food...
-
Matrix Co.'s beginning and ending inventories for the month of October are: BEGINNING ENDING DIRECT MATERIALS 67,000.00 60,000.00 WORK IN PROCESS 145,000.00 170,000.00 FINISHED GOODS 85,000.00...

Study smarter with the SolutionInn App


