Consider a solution of an unknown salt having the general formula BHCl, where B is one of
Question:
Consider a solution of an unknown salt having the general formula BHCl, where B is one of the weak bases in Table 13.3. A 0.10-M solution of the unknown salt has a pH of 5.82. What is the actual formula of the salt?
Table 13.3.
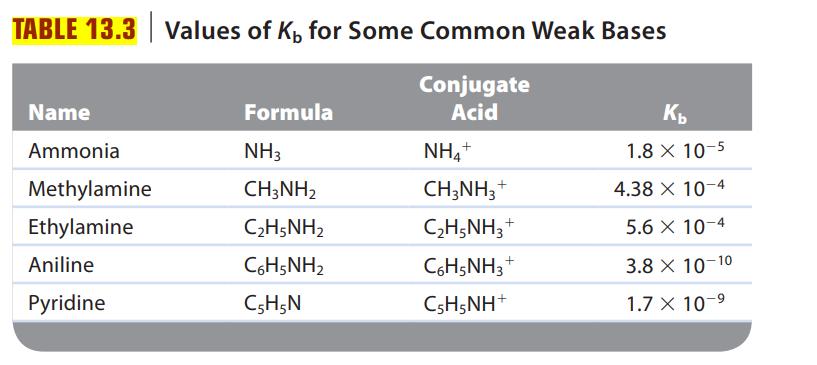
Transcribed Image Text:
TABLE 13.3 Values of K, for Some Common Weak Bases Conjugate Acid Name Ammonia Methylamine Ethylamine Aniline Pyridine Formula NH3 CH3NH₂ C2H5NH2 CoH5NH2 C5H5N NH4+ CH3NH3 + C₂H5NH3 C6H5NH3 CsH5NH* + + Kb 1.8 X 10-5 10-4 10-4 10-10 10-⁹ 4.38 x 5.6 x 3.8 X 1.7 X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the actual formula of the unknown salt we need to determine the identity of the weak ba...View the full answer

Answered By

Antony Mutonga
I am a professional educator and writer with exceptional skills in assisting bloggers and other specializations that necessitate a fantastic writer. One of the most significant parts of being the best is that I have provided excellent service to a large number of clients. With my exceptional abilities, I have amassed a large number of references, allowing me to continue working as a respected and admired writer. As a skilled content writer, I am also a reputable IT writer with the necessary talents to turn papers into exceptional results.
4.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
An aqueous solution of an unknown salt of gold is electrolyzed by a current of 2.75 amps for 3.50 hours. The electroplating is carried out with an efficiency of 90.0%, resulting in a deposit of...
-
An aqueous solution of an unknown salt of vanadium is electrolyzed by a current of 2.50 amps for 1.90 hours. The electroplating is carried out with an efficiency of 95.0%, resulting in a deposit of...
-
Separate samples of a solution of an unknown salt are treated with dilute solutions of HBr, H2SO4, and NaOH. A precipitate forms in all three cases. Which of the following cations could the solution...
-
If net assets of a business totalled 200,000 and its total assets on that date amounted to 325,000, its liabilities would amount to: (a) 125,000 (b) 200,000 (c) 525,000 (d) Not sufficient information...
-
Vickie Lynn Smith, an actress and model also known as Anna Nicole Smith, met J. Howard Marshall II in 1991. During their courtship, J. Howard lavished gifts and large sums of money on Anna Nicole,...
-
Dinitrogen pentoxide, N2O5, decomposes when heated in carbon tetrachloride solvent. N2O5 2NO2 + 1/2 O2(g) If the rate constant for the decomposition of N2O5 is 6.2 104/min, what is the half-life?...
-
As the supervisor of a group of production workers in a plant that manufactures parts for wind turbines, you have been asked by upper management to join a team of supervisors from different...
-
1. Develop numerical summaries of the data. 2. Use regression analysis to develop an estimated regression equation that could be used to predict the value score given the price of the car. 3. Use...
-
pins 12. (30 Points, 10 Pts for a, b, 5 pts for e, ) ABC Compuny manufactured jeans. In June, ABC made 1200 pair of jeans, but had hudgeted production et 100 pairs of yeam. The allocam buse for...
-
? Dean and Ellen Price are married and have a manufacturing business. They bought a piece of business equipment (7-year personal property) on 4/1/2017 for $50,000. Use half-year convention to...
-
Calculate the pH of each of the following solutions. a. 0.12 M KNO 2 b. 0.45 M NaOCl c. 0.40 M NH 4 ClO 4
-
Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka...
-
How are equivalent units used in Exhibits 21-8 and 21-9?
-
Albert is in third grade and has documented impulsivity issues in class. Develop a plan to teach Albert how to answer questions in class appropriately. He will currently shout out answers and if the...
-
What type of atmosphere is generated in the zara locations? How do the stores draw in their customers? Is there any atmospherics that would make you stay in the stores? Is it enjoyable inside, does...
-
You've been asked to create a machine learning service that helps people choose what concert to attend on a particular date based on the type of music they prefer, who is singing, and where the event...
-
What are the lessons (human resource, marketing, services, location, pricing, etc.) that Disney learned from its previous international ventures (Japan, EDL, HK)? What were some of the mistakes and...
-
17.C. a. A person asks you to convert a given point (x,y) into polar coordinates (r, 0). Explain how this might be an ambiguous question (i.e., is further information needed?). b. There is only 1 out...
-
Should employees engaged in lawful strike activity be protected from permanent replacement? Explain your reasoning.
-
Where are the olfactory sensory neurons, and why is that site poorly suited for their job?
-
Propose an efficient synthesis for the following transformation.
-
For a pair of keto-enol tautomers, explain how IR spectroscopy might be used to identify whether the equilibrium favors the ketone or the enol.
-
Acrolein is an α,β-unsaturated aldehyde that is used in the production of a variety of polymers. Acrolein can be prepared by treating glycerol with an acid catalyst. Propose...
-
Required information [ The following information applies to the questions displayed below. ] Henrich is a single taxpayer. In 2 0 2 3 , his taxable income is $ 5 3 4 , 0 0 0 . What are his income tax...
-
please FAST Question 15 1 pts Bonds & Stocks Corporation, and its officers, directors, and shareholders, buy and sell securities. SEC Rule 106-5 applies to the purchase or sale of a security by a...
-
eBook Show Me How Ofice 365 Cost of goods manufactured for a manufacturing company Two items are omitted from each of the following three lists of cost of goods manufactured statement data Work in...

Study smarter with the SolutionInn App


