How is acid strength related to the value of Ka? What is the difference between strong acids
Question:
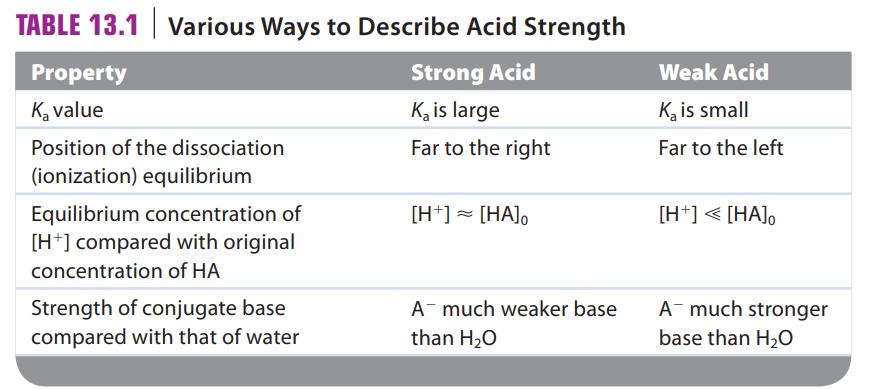
How is acid strength related to the value of Ka? What is the difference between strong acids and weak acids (see Table 13.1)? As the strength of an acid increases, what happens to the strength of the conjugate base? How is base strength related to the value of Kb? As the strength of a base increases, what happens to the strength of the conjugate acid?
Transcribed Image Text:
TABLE 13.1 Various Ways to Describe Acid Strength Strong Acid K₂ is large Far to the right Property K₂ value Position of the dissociation (ionization) equilibrium Equilibrium concentration of [H+] compared with original concentration of HA Strength of conjugate base compared with that of water [H+] = [HA]。 A much weaker base than H₂O Weak Acid K₂ is small Far to the left [H+] < [HA], A much stronger base than H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
a The acid dissociation constant Ka is a measure of how strongly an acid dissociates in waterThe hig...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Chemists know that nitric and sulfuric acids are strong acids and that acetic acid is a weak acid. They would also agree that ethanol is at best a very weak acid. Acid strength is given directly by...
-
Draw a class diagram that reflect the following Java code segment as shown in Figure 4. public abstract class Vehicle { private int numberofWheels; public Vehicle () { this (0); } protected Vehicle...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Match each of the key terms with the definition that best fits it. ________ The process of ensuring that only authorized changes are made to a system. Here are the key terms from the chapter. The...
-
What would the order of inheritance have been if Ramish had died intestate? In June 2007, Bernard Ramish set up a $48,000 trust fund through West Plains Credit Union to provide tuition for his...
-
Some lemon juice has a hydronium-ion concentration of 5.0 10-3 M. What is the pH of the lemon juice?
-
What traits do you think can help a supervisor successfully manage remote workers?
-
Prepare a table with the following headings for a monthly bank reconciliation dated September 30. For each item 1 through 12, place an x in the appropriate column to indicate whether the item should...
-
4. National Foods' ad agency has constructed the following payoff table giving its estimate of the expected profit resulting from purchasing one, two, or three advertising sports. (Another possible...
-
Let X be the winnings of a gambler. Let p(i) = P(X = i) and suppose that Compute the conditional probability that the gambler wins i , i = 1, 2, 3, given that he wins a positive amount. 1/3: p(1) %3...
-
Define or illustrate the meaning of the following terms: a. Amphoteric b. K w reaction c. K w equilibrium constant d. pH e. pOH f. pK w Give the conditions for a neutral aqueous solution at 25C, in...
-
At 35C, K = 1.6 10 -5 for the reaction If 2.0 moles of NO and 1.0 mole of Cl 2 are placed into a 1.0-L flask, calculate the equilibrium concentrations of all species. 2NOCI(g)2NO(g) + Cl(g)
-
In Problem evaluate the expression. If the answer is not an integer, round to four decimal places. 3654 36525
-
Which topics do you see as being most relevant to your current job or the job you will seek to obtain once you have earned your degree? How so ? In which ways has this course Commercial Law changed...
-
Directions Answer the following reflective questions: There do exist examples of business organizations following principles of behavior that are not entirely self-serving, but rather, are pursuing...
-
10 Count scallops cost $12.97 per pound. How much do they cost for each? A Wagyu Beef New York Strip costs $14 per pound and weighs 15 pounds. The useable yield is 12.5 pounds. How many 12 ounce...
-
How do coordinating agencies differ in a crisis, disaster, and an emergency ?Explain
-
How do we manage and respond to customer feedback and reviews to maintain a positive brand reputation? Explain with the help of examples.
-
My employer monitors my e-mail and internet usage at work and tracks my location through the GPS feature of my cell phone. I receive work- related text messages or voice mails almost every day of the...
-
Test whether the 5-year survival rate for breast cancer is significantly different between African American and Caucasian women who are younger than 50 years of age and have localized disease....
-
Draw the major product that is expected when each of the following compounds is treated with excess methyl iodide followed by aqueous silver oxide and heat: (a) Cyclohexylamine (b)...
-
Propose a synthesis for the following transformation (be sure to count the carbon atoms): Br
-
Compound A is an amine that does not possess a chirality center. Compound A was treated with excess methyl iodide and then heated in the presence of aqueous silver oxide to produce an alkene. The...
-
Plant Co completed a special landscaping job for Smith Co. Plant uses ABC and has the following activity rates: Activity Allocation Base Activity Rate Designing Number of designs $275 / design...
-
HELP ASAP On January 15, the end of the first biweekly pay period of the year, North Company's payroll register showed that its employees earned $50,000 of sales salaries Withholdings from the...
-
These account balances listed below were provided to you from the Horizon Corporation at the end of December 31, 2020. Salaries and wages payable$ 2,580 Salaries and wages expense39,850 Utilities...

Study smarter with the SolutionInn App


